Introduction
The use of airlift technology is not new in aquaculture. Airlifts "pump" water by directing the flow generated by the buoyancy of rising bubbles within a pipe or tube (Parker and Suttle 1987). Usually this action is accomplished by injecting air into the bottom of a "riser" tube (Figure 1), and as the bubbles ascend within the enclosed tube, water is drawn up with them. Airlift technology is quite simple and inexpensive, so it is preferred, when appropriate, to the standard centrifugal pump for moving water. Airlifts can be implemented within recirculating aquaculture systems as a part of the water treatment design (Parker and Suttle 1987). However, there are other uses for this simple technology. At the University of Florida, airlifts are used to collect and concentrate live food organisms fed to marine fish larvae. Airlifts are easy to construct, use, and maintain, and provide a more gentle and efficient collection alternative for fragile live feed organisms than sieving. The protocols and designs discussed in this article pertain to harvesting and feeding copepod nauplii to marine fish larvae (Ohs, Cassiano, and Rhodes 2009); however, these methods can be adapted for use with many live feed organisms.
Credit: Jason S. Broach
Design and Construction
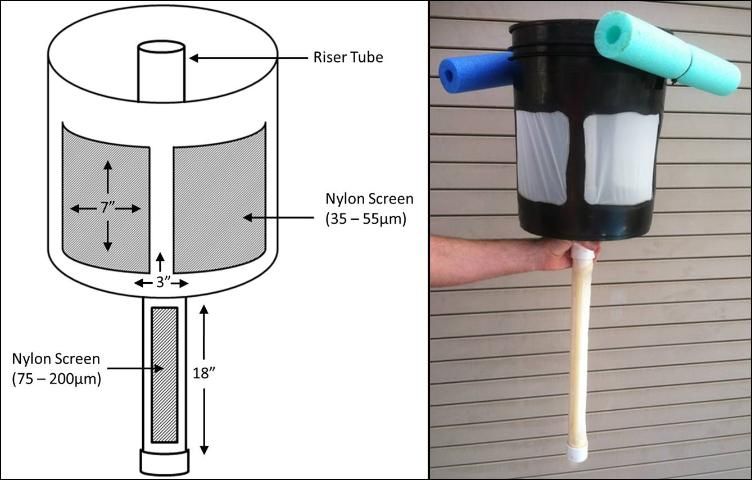
At the University of Florida, floating airlifts are used to passively separate nauplii from the rest of the copepod population. Airlift collectors for nauplii are constructed from 5-gallon plastic buckets that have had approximately two-thirds of their area removed to create 3 "windows" (Figure 1). These windows are covered with nylon mesh screen that is hot-glued in place (Figure 2). Screens are glued on the inside surface of the collector, and all seams sealed tightly to prevent nauplii from becoming trapped between the screens and the bucket. The bottom edge of the windows should be located approximately 3 inches (7.6 cm) above the bottom of the bucket. This allows water and collected nauplii to remain in the bucket after removing the collector from the copepod culture tank. A hole is drilled in the center of the bucket to allow a bulkhead fitting (1 ¼ inch diameter, threaded) to be attached. A PVC pipe (1 ¼ inch diameter, approximately 18 inches long) is then capped at one end, with the other end fitted into a male adapter. This assembly is then threaded into the bulkhead on the underside of the bucket. A window is cut out of the PVC pipe and nylon mesh screen is fitted to the window and secured with hot glue. Styrofoam, "swim noodles," or other floatation material is cut to fit around the perimeter of the bucket and attached with plastic cable ties. Inside the bucket, a PVC standpipe (1 ¼ inch diameter) is threaded into the bulkhead. It should extend approximately ¼ inch (approximately 1 cm) above the surface of the water when the collector is floating (Figure 3). A small airstone is placed inside the internal standpipe, all the way to the bottom, creating a current that pulls copepod nauplii through the screened pipe and concentrates them in the bucket. Older copepods do not pass through the screens and are not disturbed by this harvest method. The mesh size of the screen used depends on the culture species and life stage to be collected. To work properly, the screen size on the bottom incoming PVC pipe must be larger than the screen material attached to the bucket. For example, when harvesting nauplii of the calanoid copepod Parvocalanus crassirostris, a 100-micron mesh screen is used on the incoming PVC pipe and a 35-micron mesh screen is used on the bucket windows. It should be noted that this design has been optimized to collect copepod nauplii from large (50–90 gal) culture vessels. Collectors may be altered as needed to accommodate a variety of tank sizes and live feed organisms.

Credit: Jason S. Broach

Credit: Jason S. Broach
Operation and Enumeration
When collection is required, an airlift collector is put into the appropriate copepod culture tank and allowed to float freely. Air flow rate is then adjusted so the water is flowing up the standpipe of the airlift collector. It is important to make sure that the water flow is strong enough to bring the water up and over the lip of the standpipe and into the bucket. However, if the flow is too strong, the collector may quickly clog and not function properly. It is also best to collect nauplii prior to feeding the copepod culture as any flocculent material in the water can cause the screens to clog and impede nauplii collection.
The airlift can now operate until it is retrieved from the copepod culture tank. At the University of Florida, we routinely let the collector operate for at least an hour (see "Airlift Efficiency"), though we have let it run for as long as 4 hours. Once finished, the collector is removed from the copepod culture tank, and the water inside the bucket, which contains the collected nauplii, is poured into a graduated container via the side of the bucket where a window was not cut out. The density of the copepod nauplii in the graduated container can then be determined volumetrically by counting subsamples. Once the density is determined, the total number harvested can be estimated by counting nauplii in a small volume of water and multiplying the number of nauplii in the sample by the volume of the graduated container. For example, if the volume of your graduated container is 1 liter (1000 milliliters, mL), then counting the number of nauplii in a 2 mL subsample and multiplying that number by 500 would give you an estimate of the total number of nauplii in your 1 liter graduated container. If desired, the nauplii are then ready for feeding or prefeeding treatments such as enrichment or disinfection.
Maintenance
Occasional maintenance should be performed on the collectors to ensure reliable performance. The screens should be checked for holes and tears routinely and may also need to be replaced over time as the size of the mesh can become distorted and irregular due to general wear and tear. The glue may also become loose and needs to be checked periodically. Any one of these issues will either let larger copepods into the collection bucket, quickly clogging the screens, or allow collected nauplii to exit the collection bucket and return to the culture population, either of which will reduce the efficacy of the collector. Flotation material will also need to be routinely inspected and replaced depending upon the durability of material used. After each use, the collector should be washed with fresh water and left to dry. It is also best to store the collector out of the sunlight because light will quickly degrade the screens and flotation materials.
Airlift Efficiency
Two sizes of airlift collectors have been tested for efficiency: a 2-gallon bucket size and a 5-gallon bucket size (Cassiano 2009). Both used the same design as described above and differed only in the size of bucket. A simple experiment was conducted to see which size airlift harvested the greater percentage of copepod nauplii from a 53-gal culture tank after one hour of airlift operation. Our results indicated the 5-gallon bucket airlift (90.3 ± 4.7%) was more efficient than the 2-gallon bucket airlift (60.6 ± 2.5%) for harvesting a culture tank of this size. Undoubtedly, the size and shape of each culture container will influence the specific collector chosen. This study does display, however, that within one hour of operation, 90% or greater of the nauplii within a culture vessel can be harvested using airlift collectors.
Conclusion
Airlifts provide a simple and efficient means to transport water within aquaculture systems. By adapting this technology, we were able to passively collect copepod nauplii from their culture tanks with little detrimental effect on the non-target population. Alternative methods for copepod nauplii collection using a broad array of equipment have proven successful (Payne and Rippingale 2001; Kline, Callan, and Laidley 2014), but airlift collectors provide several advantages over most of these. Airlift collectors are simple to make, use very little electricity, and are easily adapted to a variety of culture scenarios. Airlift technology may also be used for harvest of other live-feed organisms such as rotifers and ciliates, or it may be modified to collect pelagic fish eggs from broodstock tanks in aquaculture facilities and public aquariums (Cassiano et al. 2014; Ohs et al. 2019). If properly constructed and operated, airlift collectors can greatly reduce labor in an aquaculture setting. They are simple tools that have a variety of uses and that can easily be amended to accomplish an assortment of tasks.
References
Cassiano, E. J. 2009. "Evaluation of Pseudodiaptomus pelagicus as a First Feed for Florida Pompano, Trachinotus carolinus, Larvae." Master's thesis, University of Florida.
Cassiano, E. J., M. L. Wittenrich, T. B. Waltzek, N. K. Steckler, K. P. Barden, and C. A. Watson. 2014."Utilizing Public Aquariums and Molecular Identification Techniques to Address the Larviculture Potential of Pacific Blue Tangs (Paracanthurus hepatus), Semicircle Angelfish (Pomacanthus semicirculatis), and Bannerfish (Heniochus sp.)." Aquaculture International 23(1): 253–265.
Kline, M. D., C. K. Callan, C. W. and Laidley. 2014. "Advances in Intensive Copepod Production Technology." Global Aquaculture Advocate 17(1): 68–70.
Ohs, C. L., E. J. Cassiano, and A. Rhodes. 2010. Choosing an Appropriate Live Feed for Larviculture of Marine Fish. FA167. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/fa167
Ohs, C.L., J.S. Broach, E.J. Cassiano, and J. Marcellus. 2019 Design and Operation of Egg Collectors for Pelagic Spawning Marine Fishes. FA211. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/fa211
Parker, N. C., and M. A. Suttle. 1987. "Design of Airlift Pumps for Water Circulation and Aeration in Aquaculture." Aquacultural Engineering 6(2): 97–110.
Payne, M. F., and R. J. Rippingale. 2001. "Intensive Cultivation of the Calanoid Copepod Gladioferens imparipes." Aquaculture 201: 329–342.