This document is one in a series on ocean acidification (OA). The series introduction, Ocean Acidification: An Introduction (https://edis.ifas.ufl.edu/fa206), contains information on the causes and chemistry of OA. Because OA is very large-scale and complex, each document in the series addresses a specific aspect of this issue. Florida, with an extensive coastline and deep cultural and economic ties to marine resources, will be directly affected by changes in seawater chemistry. Thus, each topic in the series also highlights information of specific relevance for Florida.
Introduction
Ocean acidification is the ongoing decrease in ocean pH associated with the uptake of excess carbon dioxide (CO2) from the atmosphere. This publication will focus on the spatial and temporal variability in ocean pH and provide an overview of pH variability in the coastal waters of Florida.
The absorption of atmospheric CO2 by the world's oceans has changed the chemical properties of seawater. These changes have caused the ocean pH and calcium carbonate mineral saturation state to decline. pH is a chemistry measurement scale that expresses the acidity or alkalinity of a solution. Calcium carbonate saturation state describes the degree to which calcium carbonate will form or dissolve in seawater. Calcium carbonate is necessary for marine calcifying organisms to build their shells and skeletons. Conditions favorable for calcium carbonate formation are considered saturated, whereas those that favor dissolution are considered undersaturated. Of the two mineral forms of calcium carbonate, aragonite and calcite, aragonite is the more easily dissolved form and is more likely to become undersaturated, or lowered, due to OA.
pH and aragonite saturation states are naturally variable throughout the global ocean in both time and space. This variability is determined by a number of biological (e.g., photosynthesis and respiration), chemical (e.g., dissolved oxygen), and physical (e.g., light availability, temperature, and mixing) forces. Because of this natural variability, predicting how pH and aragonite saturation states will change due to ocean acidification is a challenge. Natural variability is also one of the factors that makes it difficult to predict how OA will affect marine organisms from laboratory studies. In order to predict and understand changes in carbonate chemistry due to ocean acidification, scientists must separate this natural variability from changes due to anthropogenic CO2. However, over long-term time scales, as atmospheric levels of CO2 continue to rise and oceans continue to absorb excess CO2, decreases in pH and aragonite saturation state are expected to escalate.
Temporal Variability
In coastal and estuarine waters, natural variability in pH can occur on a multitude of scales ranging from hourly (tidal), daily, and monthly (seasonal) time frames. The primary drivers of this temporal variability are biological processes associated with ecosystem metabolism—photosynthesis and respiration (Silbiger and Sorte 2018). When organisms photosynthesize, they remove CO2 from the water and release oxygen. Taking CO2 out of the water on a large enough scale can result in measurable increases in pH. For example, it has been suggested that photosynthesis of seagrass meadows in shallow waters might provide refuge from OA for other organisms living nearby (Manzello et al. 2012; Yates et al. 2016). However, cellular respiration has the opposite effect on CO2 concentrations and decreases pH. On net, communities that are dominated by photosynthesizing organisms remove CO2 from the ocean, although strong and predictable diel (day/night) pH cycles are readily observed. Conversely, consumer-dominated communities show decreases in pH due to constant respiration (Shaw et al. 2012, Silbiger and Sorte 2018). On larger timescales, this type of biologically driven pH variability can be observed seasonally because high levels of photosynthesis in the summer are associated with high light availability. Increased photosynthesis leads to pH increases while lower productivity in winter causes lower pH. These seasonal changes can be seen along the Florida Reef Tract, where seasonal changes in net photosynthesis and respiration lead to fluctuations in aragonite saturation state, calcification and dissolution of coral skeletons, and pH (Muehllehner et al. 2016). These community-dominated influences are important when considering the impacts of ocean acidification at local scales. In coastal waters, the variability in pH over short and long timescales may provide a buffer to OA because organisms are already adapted to extremes; however, disruptions and shifts in community structure can make even coastal organisms more vulnerable to OA.
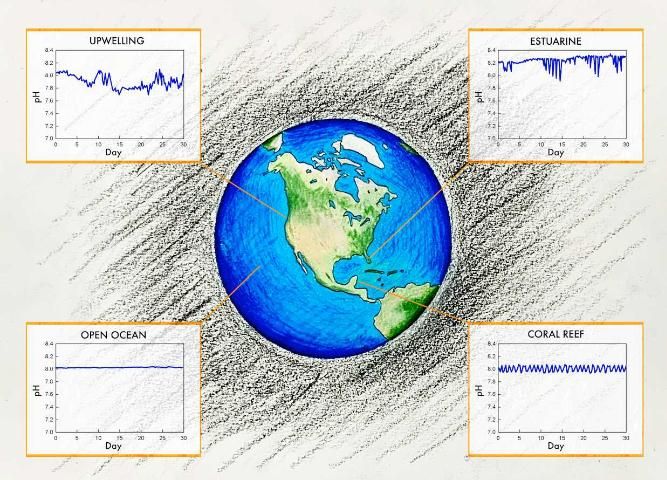
Spatial Variability
Using results from field, laboratory, and modeling studies, scientists can estimate global changes in water chemistry. As discussed above, estuarine, near-shore, and coastal upwelling locations can experience a lot of natural variability in pH and aragonite saturation, whereas the open ocean is more stable (Duarte 2013, Hoffman et al. 2011). However, studies of the open ocean indicate that most of the ocean will continue to acidify, though the rates will vary by region.
Credit: Joseph Henry, UF/IFAS
Excess CO2 enters the ocean at the surface through diffusion. Stratification of ocean waters keeps most of the CO2 in the upper portions of the ocean; therefore, the impacts of OA are most pronounced in the layer <500 m deep. The equatorial regions between 20°S and 20°N have the lowest surface pH, but this area is expected to experience the smallest changes in pH because it is already supersaturated with CO2. Conversely, the high-latitude polar regions have experienced and will continue to experience the largest changes in pH due to lower buffering capacity (Jiang et al. 2019). At the surface, aragonite saturation states are generally highest in the tropics and lowest in the high latitudes. Aragonite saturation is strongly linked to sea surface temperature, and higher temperatures lead to higher saturation rates (Jiang et al. 2019).
Upwelling zones are areas where deep, cold waters are brought up to the surface through wind and circulation patterns. The impacts of OA in upwelling zones are spatially dependent. In equatorial upwelling zones, upwelling waters tend to be low in anthropogenic CO2. Consequently, these waters buffer the impacts of OA in surface waters and slow down the decrease in pH. The upwelling zone along the west coast of the United States, however, is particularly vulnerable to OA due to a combination of natural and human-induced factors. The upwelling waters come from the mid-shelf region (100–300 m from the surface) and are naturally high in CO2 due to the historic accumulation of CO2 produced as a byproduct of respiration processes associated with the breakdown of sinking organic matter. In addition to having a naturally lower pH (i.e., more acidic) and aragonite saturation state as well as a reduced buffering capacity, the upwelled water is low in dissolved oxygen and high in nutrients and dissolved organic carbon. As a result, this upwelling zone will likely experience accelerated OA and is especially vulnerable (Gruber et al. 2012, Chan et al. 2019). This does not mean, however, that impacts will be greatest in these regions, because organisms may already be suited for these relatively stressful conditions.
Less information is known about the nearshore, coastal regions where, due to both natural and human influences, pH and carbonate chemistry variability are greater than those same variabilities in the open ocean. Changes in seawater chemistry associated with human-induced inputs are so significant that these impacts have been termed coastal acidification. These coastal inputs include but are not limited to freshwater riverine inputs, atmospheric deposition, nutrient inputs, and organic matter inputs. In coastal and estuarine waters, CO2 produced by internal loading is much greater that the atmospheric CO2 associated with OA. This is especially true for nutrient-enhanced eutrophic waters. The impacts of coastal acidification are similar to those of OA, but local pollution management can lead to significant improvements that may also help make a system more resilient to OA.
Ocean Acidification in Florida
Scientific studies suggest that sites along the Florida coast and in the Greater Caribbean Regions have had significant changes in pH, salinity, temperature, and aragonite saturation over the past 20 years (Gledhill et al. 2008). Due to the complexities associated with coastal carbonate chemistry, however, it is not known whether these changes are the result of elevated CO2 concentrations or other factors. Regardless, they are likely to have significant impacts on Florida's economic and environmental resources. Below is an overview of some ocean acidification impacts in Florida.
Estuaries in Florida have demonstrated the pH fluctuations typical of highly productive nearshore environments. Monthly sampling in Florida Bay showed extensive variation in pH (7.85–8.10) and Florida and Tampa Bays exhibited average diurnal differences (over 3 days) in pH of 0.22 units (Yates et al. 2007). Annual sampling in St. Joseph Bay, another seagrass-dominant subtropical Florida estuary, showed ranges in monthly pH values of 7.36–8.29 with an average pH of 7.84, significantly less than the global open ocean average of 8.10 (Challener et al. 2016). These studies highlight the challenges in conducting valid coastal ocean acidification experiments in areas where natural fluctuation is so great. Even still, coastal acidification is expected to continue in these areas, though there is large uncertainty as to the variability in region and time. Regardless, they are likely to have significant impacts on Florida's economic and environmental resources.
Corals are expected to be especially vulnerable to OA because of their calcium carbonate skeletons and the decline in the saturation state of carbonate minerals (refer to FA220: Ocean Acidification: Calcifying Marine Organisms). Florida is home to the only nearshore coral reef in the continental United States. As such, the Florida Reef Tract (FRT) has been one of the most studied ecosystems in regard to OA impacts within the state of Florida. A 2016 study of the FRT confirms the vulnerability of Florida's corals to ocean acidification. Northern portions of the coral reef tract are now "net erosional," which means that in these areas more calcium carbonate is being dissolved than is being created by biological activity each year. This suggests that the FRT is no longer a functioning, healthy coral reef (Muehllehner et al. 2016). Furthermore, a 2016 study of two Caribbean species examined the idea that corals that occur in relatively shallow water may be resilient to the effects of OA. Despite experiencing daily fluctuations in pH, the results suggested that even corals acclimatized to areas with high natural variability in pH were harmed or killed by the pH levels predicted for the year 2100 (Camp et al. 2016).
Florida has some of the most extensive seagrass habitat in the world, and these areas are some of our most important marine ecosystems. Seagrass meadows are also one of the most productive ecosystems. Vast seagrass meadows can sequester carbon through capture and storage from the atmosphere and serve as a net sink of carbon dioxide. Macroalgae, though more diverse, have received less attention than seagrasses. Noncalcifying macroalgae are also ecologically important and may become dominant in areas experiencing significant loss in corals and calcifying macroalgae. As atmospheric and oceanic CO2 concentrations continue to increase, organisms such as seagrasses and noncalcifying marine algae may indeed be "winners" and may actually help to buffer the impacts of ocean acidification in certain areas. Higher numbers of species affected by OA, regardless of whether the effects are positive or negative, increase the likelihood of major shifts in ecological function of marine systems (Kleypas and Yates 2009).
Conclusion
Variability in ocean pH across space and time is extremely nuanced and complex. The primary objective of this document is to increase awareness of these issues and understanding of how they relate to OA. The marine environment is not static or uniform, and how we think about OA must include consideration of pH variability and the uncertainties involved. Regardless, mounting evidence suggests that OA will have negative consequences in a variety of marine environments on different timescales. A lack of absolute certainty about what these consequences will be should not preclude actions to reduce carbon emissions on local, regional, and global scales and thereby diminish the severity of future acidification in our oceans.
References
Camp, E. F., D. J. Smith, C. Evenhuis, I. Enochs, D. Manzello, S. Woodcock, and D. J. Suggett. 2016. "Acclimatization to high-variance habitats does not enhance physiological tolerance of two key Caribbean corals to future temperature and pH." Proceedings of the Royal Society B: Biological Sciences 283: p.20160442. doi:10.1098/rspb.2016.0442
Challener, R. C., L. L. Robbins, and J. B. McClintock. 2016. "Variability of the carbonate chemistry in a shallow, seagrass-dominated ecosystem: implications for ocean acidification experiments." Marine and Freshwater Research 67: 163–172. doi:10.1071/MF14219
Chan, F., J. A. Barth, K. J. Kroeker, J. Lubchenco, and B. A. Menge. 2019. "The dynamics and impact of ocean acidification and hypoxia." Oceanography 32(3): 62–71. doi: 10.2307/26760084.
Gledhill, D. K., R. Wanninkhof, F. J. Millero, and M. Eakin. 2008. "Ocean acidification of the greater Caribbean region 1996–2006." Journal of Geophysical Research: Oceans 113: C10031. doi:10.1029/2007JC004629
Gruber, N., C. Hauri,, Z Lachkar, D. Loher, T. L. Frölicher, and G. Plattner. 2012. "Rapid progression of ocean acidification in the California Current System" Science 337: 220–223. doi:10.1126/science.1216773
Guinotte, J. M., and V. J. Fabry. 2008. "Ocean acidification and its potential effects on marine ecosystems." Annals of the New York Academy of Sciences,1134: 320–342. doi:10.1196/annals.1439.013
Jiang, L., B. R. Carter, R. A. Feely, S. K. Lauvset, and A. Olsen. 2019. "Surface ocean pH and buffer capacity: past, present and future." Nature 9: 18624. doi:10.1038/s41598-019-55039-4.
Kleypas, J. A., and K. K. Yates. 2009. "Coral reefs and ocean acidification." Oceanography 22: 108–117.
Manzello, D. P., I. C. Enochs, N. Melo, D. K. Gledhill, and E. M. Johns. 2012. "Ocean acidification refugia of the Florida Reef Tract." PloS One 7: e41715. doi:10.1371/journal.pone.0041715
Muehllehner, N., C. Langdon, A. Venti, and D. Kadko. 2016. "Dynamics of carbonate chemistry, production, and calcification of the Florida Reef Tract (2009–2010): Evidence for seasonal dissolution." Global Biogeochemical Cycles 30: 661–688. doi:10.1002/2015GB005327
Shaw, E. C., B. I. McNeil, and B. Tilbrook. 2012. "Impacts of ocean acidification in naturally variable coral reef flat ecosystems." Journal of Geophysical Research: Oceans 117: C03038. doi:10.1029/2011JC007655
Silbiger, N. J., and C. J. B. Sorte. 2018. "Biophysical feedbacks mediate carbonate chemistry in coastal ecosystems across spatiotemporal gradients." Nature 8: 796. doi: 10.1038/s41598-017-18736-6
Yates, K. K., C. Dufore, N. Smiley, C. Jackson, and R. B. Halley. 2007. "Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay." Marine Chemistry 104: 110–124. doi:10.1016/j.marchem.2006.12.008
Yates, K. K., R. P. Moyer, C. Moore, D. Tomasko, N. Smiley, L. Torres-Garcia, C. E. Powell, A. R. Chappel, and I. Bociu. 2016. "Ocean acidification buffering effects of seagrass in Tampa Bay." In Proceedings of the 6th Tampa Bay Area Scientific Information Symposium (BASIS) 6: 273–284. https://pdfs.semanticscholar.org/15b5/0229d8e2963516e928b9f288c34c869e263d.pdf