Introduction
The Orchidaceae are one of the largest families of flowering plants in the world with almost 30,000 species (Brown 2005). Selection pressure on some orchid taxa (groups of related orchids) has resulted in the evolution of dramatic morphological (physical and structural) differences among orchid lineages (Kew Royal Botanic Gardens 2013). One unique trait that has evolved in orchids is the fusion of the stamens (all male reproductive parts—anthers and filaments) with the pistil (all female reproductive parts—stigma, style, and ovary) in the flower. This reduction in stamen number has led to orchid groups with only three, two, or one stamen(s). More than 99% of all described orchid species have only one stamen in the flower, which is a characteristic feature of the Orchidaceae (Kew Royal Botanic Gardens 2013).
Most orchid flowers have the same basic reproductive structures (Figure 1). A central structure known as the column is a unique adaptation of orchids that houses both the male (anther) and female (stigma) parts of the flower (Roberts and Dixon 2008). The column—at least the distal (away from the center) portion—is oriented horizontally. The anther is located at the distal end of the column. The stigma is located near the distal end and just on the underside of the column. Directly below the column is the labellum, or lip, a modified petal that acts as a landing area for pollinators (Brown 2005).
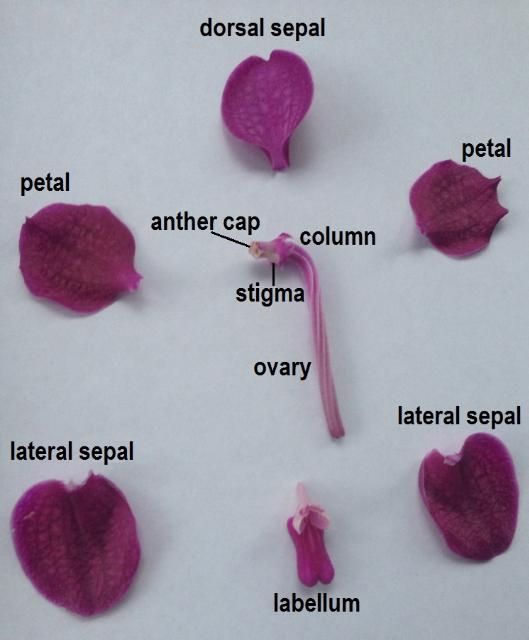
Credit: Haleigh Ray, UF/IFAS
When present, the floral nectaries (nectar producing tissue on the plant) are typically located at the base of the labellum or as a nectar spur behind the flower. As a pollinator moves on the labellum, it comes into contact with the pollen. Unlike the loose—and often wind-dispersed—pollen grains of most flowering plant families, orchid pollen grains are fused together into compact structures called pollinia. The pollinia (two to eight knob-like packets of pollen) are located under the male anther cap and contain a sticky structure called the viscidium (Figure 2), which helps the pollinia adhere to a pollinator as it feeds or seeks to mate with the column (Roberts and Dixon 2008). When a pollinator visits another flower, the pollinia are likely transferred to the stigma.
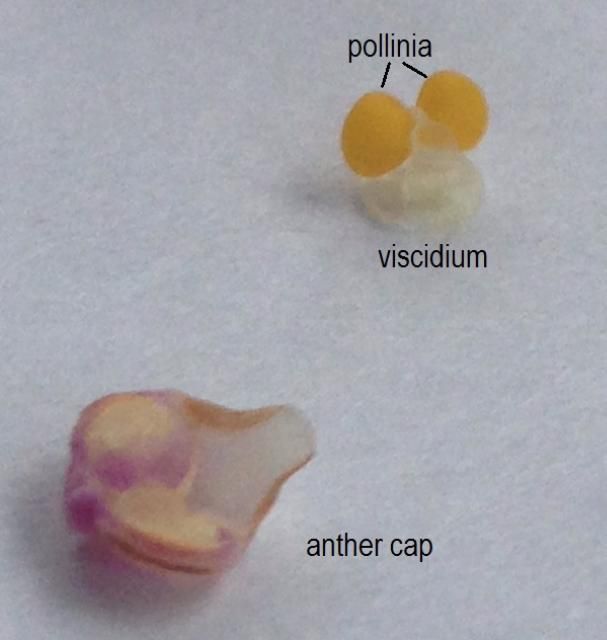
Credit: Haleigh Ray, UF/IFAS
If pollination is successful, a pollen tube will develop and grow, and the ovary of the flower will begin to swell and eventually form a seed capsule filled with hundreds of thousands to millions of seeds (Roberts and Dixon 2008). After the seed capsule matures, seeds are released and disseminated by the wind until they come in contact with a substrate (surface on which the plant lives) for germination. However, orchid seeds lack endosperm, which is the nutritive tissue that sustains embryo development and seed germination. Therefore, orchid seeds need to associate with mychorrizal fungi, which help the seed absorb water and nutrients and convert cellulose and other complex molecules in the substrate into simple sugars needed by the orchid. Consequently, all members of the Orchidaceae produce seeds that rely on a substrate with mycorrhizal fungi to complete their life cycle in situ (Rasmussen 1995).
Mycorrhizal associations are variable among orchids. Epiphytes (plant harmlessly attached to another plant) may only use the fungus in the seed germination phase, with the adults relying on nutrient uptake from rainfall and runoff, while terrestrials (living in the ground) may have a high level of continued mycorrhizal dependency (Roberts and Dixon 2008). In order for an epiphytic orchid to germinate, a mycorrhizal fungus must be present in the area where the wind-dispersed seed lands.
How Orchids Are Pollinated
Orchidaceae flowers are pollinated by diverse vertebrate and invertebrate taxa. Bees and wasps (order Hymenoptera) are common orchid pollinators, though organisms in the orders Lepidoptera, Diptera, Coleoptera, and others also pollinate orchids, as do some small birds (Statman-Weil 2001, Hawkeswood and Turner 2004, Lehnebach and Robertson 2004, Micheneau et al. 2010, Newman et al. 2011, Stökl et al. 2011).
There are many different kinds of orchid-pollinator relationships. Some orchid species are pollinated by numerous taxa, while others are specialized and are pollinated by only one pollinator species. Orchids that have specialized relationships with just one pollinator species often have more efficient pollen transfer than orchids that rely on multiple general pollinators. Specialization allows direct transfer of pollen to the same orchid species and reduces the chance that the pollinia will be dropped or transferred to the wrong orchid species (Scopece et al. 2010). Also, specialization may involve the attachment of pollen to one specific location on the pollinator, ensuring that even if the pollinator visits another species, the pollinia likely will not be transferred until the pollinator revisits an orchid of the same species that donated the pollen (Scopece et al. 2010).
Methods of Attraction
Because orchids are so diverse, the pollination systems are highly variable and can be extremely specialized or complex. Many orchids do not rely on the pollinator's quest for nectar but use some form of deception to attract pollinators, often by physical appearance, chemical cues, or a combination of both (Wong and Schiestl 2002).
Ophrys apifera, or the bee orchid (Figure 3), is the most familiar example of an orchid that uses the physical appearance of its flowers to deceive pollinators. The lip of this species' flower resembles a female of a species of solitary bee (Eucera spp.; Hymenoptera; Anthophoridae) sitting on the flower. Male solitary bee species attempt to copulate with the flower and eventually move on in search of other females. During these mating attempts, the pollinium attaches to the male bee. When the bee is deceived by another flower and attempts to mate with it, the pollinium is transferred to the other flower's pollen receptor (stigma), resulting in pollination (Devey 2008). This is also referred to as pseudocopulation.

Credit: BerndH (http://creativecommons.org/licenses/by-sa/3.0/)
Drakaea orchids (Figure 4) are an example of an orchid that uses a combination of physical and chemical cues. The flowers of this orchid resemble female wasps in the genus Zaspilothynnus (Tiphiidae) and release a chemical that mimics a mating pheromone of this female wasp. As the males attempt to mate with the flowers, they contact the pollinia. When they begin this process with the next flower, the pollen in transferred.

Credit: Myles H. M. Menz, Ryan D. Phillips, Kingsley W. Dixon, Rod Peakall, and Raphael K. Didham (http://creativecommons.org/licenses/by/2.5/)
Some orchids attract insect pollinators by releasing compounds that resemble the pheromones of a mate, or by excreting a chemical that can be collected by a pollinator and used for defense or mate attraction (Wong and Schiestl 2002). One example of this kind of orchid is Epidendrum paniculatum. It releases pyrrolizidine alkaloids (PAs) that male lepidopterans in the moth subfamily Arctiinae and nymphalid butterfly subfamilies Ithomiinae and Danainae use for both seeking mates and defense (DeVries and Stiles 1990). DeVries and Stiles (1990) surveyed E. paniculatum in Costa Rica along with PA baits and found that 98% of the insects attracted to the flowers and baits were male lepidopterans that use PA for mate attraction and defense against predators.
In addition to deception, orchids may use traps, triggers, or false nectar rewards to attract pollinators. Orchids in the genus Coryanthes, or bucket orchids, use a trap method for pollination. These orchids have a modified labellum that forms a bucket (Figure 5). The flower secretes a fluid into these buckets that attracts male euglossine bees. The bees have a pouch-like structure on their hind legs in which they store the oil secreted from these orchids. This oil is later used in courtship to attract female mates. When the bees fly to collect the oil, they become trapped in the bucket. There is only one part of the flower that allows the bees to escape, and as the bees extricate themselves from the trap, they are forced to come in contact with the pollinia. They are temporarily trapped while the pollinia are secured to the bees thorax; then, when the bee collects oil from yet another flower, the pollinia are transferred to it. As a result, this mechanism ensures cross-pollination.

Credit: Orchi, (http://creativecommons.org/licenses/by-sa/3.0/)
While some orchids use deceit to initiate pollination, others have evolved other very specialized relationships with their insect pollinators. The orchid Angraecum sesquipedale, or Darwin's orchid, and Dendrophylax lindenii, or ghost orchid (Figure 6), which is native to south Florida, both require a specific lepidopteran pollinator. These orchids have a long nectar spur that only a hawk moth is able to reach with its long proboscis (elongated sucking mouthpart) (Kritsky 1991).

Credit: Haleigh Ray, UF/IFAS
Orchid Pollinator Taxa
Though the examples thus far have detailed hymenopteran and lepidopteran pollinators, other orders of insects have been documented as orchid pollinators as well.
As orchid pollinators, dipterans are less important than the previously discussed insect orders, though several dipteran-pollinated orchid species exist. One example is the orchid Epipactis veratrifolia, which is pollinated by hoverflies (also known as flower flies; Syrphidae), whose larvae feed on aphids. This flower releases a chemical compound that mimics an aphid alarm pheromone, signaling to the adult female hoverfly that there would be a food supply for her offspring (Stökl et al. 2011). When the hoverfly female arrives at the flower searching for aphids and a place to oviposit (lay eggs), she comes in contact with the pollinia. This often results in females laying their eggs in the flower of the orchid (Stökl et al. 2011).
There are also several families of Coleoptera (Hawkeswood and Turner 2004) and one orthopteran, an undescribed species of raspy cricket in the family Gryllacrididae, that have been found to pollinate orchids. Researchers from Kew Royal Botanic Gardens discovered the raspy cricket pollinating an Angraecum orchid on Réunion Island, off the coast of Madagascar (Micheneau et al. 2010).
Though they are the most frequent orchid pollinators, insects are not the only taxon to provide this service. Hummingbirds and snails also may affect pollination. Although only about 3% of orchids are hummingbird pollinated, the total number of orchid species pollinated by hummingbirds is still approximately 1,000 species (Siegel 2011). Orchids that rely on hummingbirds for pollination are brightly colored and often do not have a fragrance. Many of the orchids pollinated by hummingbirds will produce dark pollinia in shades of gray, blue, or brown. These colors are similar to the colors of hummingbirds' beaks, thus reducing the chance of the bird noticing the pollinia and cleaning the pollinia off its beak when grooming (Siegel 2011).
Autogamy
It is also possible for orchids to reproduce without the use of a pollinator. In spontaneous autogamous systems (auto-pollination), the pollinia become located on the stigma and pollination occurs in the absence of a pollinator. This process occurs through several mechanisms including powdery pollinia, expansion of the pollinia, or a deformation of part of the column (Catling 1990, Gale 2007). Through autogamy, orchid species that exhibit this trait do not need to rely on a suitable pollinator. It is thought that autogamy facilitates range expansion of a plant into areas where pollinators are rare or not present, and autogamy may be the consequence of range expansion (Catling 1990). Fenster and Martén-Rodriguez (2007) suggest that autogamy may be more widespread than previously believed and that auto-pollination may have evolved alongside self-pollination to adapt to selection pressures. These pressures include pollinator limitation, as auto-pollination can provide reproductive insurance against the absence of specialized orchid pollinators (Fenster and Martén-Rodriguez 2007).
Autogamy is not uncommon in the Orchidaceae (Johnson et al. 2009). More than 350 orchid species have been reported to exhibit autogamy, and it is estimated that at least 20% of species found in Ecuador and Puerto Rico are autogamous (Ackerman 1983, Catling 1990). Several species of orchids found in southern Florida have been observed to exhibit autogamy. In these reports, seed capsule formation was not investigated, making it difficult to determine if autogamy is an advantageous method of seed capsule production in regards to quality and number of seeds produced (Goss 1973, Johnson et al. 2009). A study by Johnson et al. (2009) examined the breeding system of a Florida terrestrial orchid Eulophia alta (Figure 7), which has been reported to be autogamous. In those occurrences of auto-pollination, they found that only 7.1% of the flowers formed seed capsules, and these data indicated that autogamy is rare in E. alta (Johnson et al. 2009). This study suggests that the capsules formed naturally in the field may result from pollination events that were not observed.

Credit: João Medeiros (http://creativecommons.org/licenses/by/2.0/)
Future Research
The interactions between orchids and their pollinators are diverse and often complex. Much research is required to fully understand these interactions, and the pollinators for many orchid species are still unknown. With close to 30,000 species of orchids, there are likely numerous other taxa of both insect and non-insect pollinators. Orchids use an extremely wide variety of known methods to achieve pollination, commonly involving either chemical or visual cues including mimicry. These characteristics help demonstrate how orchids have become so varied and make orchids a model system for examining floral adaptation and diversity.
References
Ackerman, J. D. 1983. "Diversity and seasonality of male Euglossine bees (Hymenoptera: Apidae) in central Panama." Ecology 64: 274–283.
Brown, P. M. 2005. Wild Orchids of Florida: With References to the Atlantic and Gulf Coastal Plains. Gainesville, FL: University Press of Florida, Gainesville, 432.
Catling, P. M. 1990. "Auto-pollination in the Orchidaceae." In Orchid Biology: Reviews and Perspectives, edited by J. Arditti, 121–158. Portland, OR: Timber Press.
Devey, D. 2008. "Ophrys: A case of the deceitful origin of species." Kew Scientist 33: 1.
DeVries, P. J., and F. G. Stiles. 1990. "Attraction of Pyrrolizidine Alkaloid Seeking Lepidoptera to Epidendrum Paniculatum Orchids." Biotropica 22: 290–297.
Fenster, C. B., and S. Martén-Rodríguez. 2007. "Reproductive assurance and the evolution of pollination specialization." International Journal of Plant Sciences 168: 215–228.
Gale, S. 2007. "Autogamous seed set in a critically endangered orchid in Japan: Pollination studies for the conservation of Nervilia nipponica." Plant Systematics and Evolution 268: 59–73.
Goss, G. J. 1973. "Pollination biology in the Orchidaceae: Polystachia flavescens, Epidendrum difforme, and Eulophia alta from south Florida; Encyclia gracilis, Encyclia altissima,and Encyclia rufa from Great Inagua, Bahamas." Master's thesis, Florida Atlantic University.
Hawkeswood, T. J. and J. R. Turner. 2004. "Observations on Metriorrhynchus rhipidius (Macleay) (Coleoptera: Lycidae) feeding on nectar from the flowers of Leucopogon muticus R. Br. (Epacridaceae) at Kenthurst, New South Wales, Australia." Calodema 2: 8–10.
Johnson, T. R., S. L. Stewart, P. Kauth, M. E. Kane, and N. Philman. 2009. "Confronting assumptions about spontaneous autogamy in populations of Eulophia alta (Orchidaceae) in south Florida: Assessing the effect of population treatments on seed formation, seed germination and seedling development." Botanical Journal of the Linnean Society 161: 78–88.
Kew Royal Botanic Gardens. 2013. "Why should we study orchids?" Kew Royal Botanic Gardens. http://www.kew.org/science/orchids/whystudy.html?words=Breivik (April 2015)
Kritsky, G. 1991. "Darwin's Madagascan Hawk Moth Prediction." American Entomologist 37: 206–210.
Lehnebach, C. A. and A. W. Robertson. 2004. "Pollination Ecology of Four Epiphytic Orchids of New Zealand." Annals of Botany 93: 773–781.
Micheneau, C., J. Fournel, B. H. Warren, S. Hugel, A. Gauvin-Bialecki, T. Pailler, D. Strasberg, et al. 2010. "Orthoptera, a new order of pollinator." Annals of Botany 105 (3): 355–364, doi:10.1093/aob/mcp299.
Newman, E., B. Anderson, and S. D. Johnson. 2011. "Flower colour adaptation in a mimetic orchid." Proceedings of the Royal Society B: Biological Sciences 279: 2309–2313.
Rasmussen, H. N. 1995. Terrestrial Orchids from Seed to Mycotrophic Plant. Cambridge, U.K.: Cambridge University Press.
Roberts, D. L., and K. W. Dixon. 2008. "Orchids." Current Biology 18: 325–329.
Scopece, G., S. Cozzolino, S. D. Johnson, and F. P. Schiestl. 2010. "Pollination Efficiency and the Evolution of Specialized Deceptive Pollination Ssystems." The American Naturalist 175: 98-105.
Siegel, C. 2011. "Orchids and Hummingbirds: Sex in the Fast Lane." Orchid Digest 75: 8–17.
Statman-Weil, Z. 2001. "Aedes communis: The Pollinating Mosquito." US Forest Service. https://www.fs.usda.gov/wildflowers/pollinators/pollinator-of-the-month/aedes_communis.shtml (February 2023)
Stökl, J., J. Brodmann, A. Dafni, M. Ayasse, and B. S. Hansson. 2011. "Smells like aphids: Orchid flowers mimic aphid alarm pheromones to attract hoverflies for pollination." Proceedings of the Royal Society B: Biological Sciences, doi:10.1098/rspb.2010.1770.
Wong, B. M. and F. P. Schiestl. 2002. "How an orchid harms its pollinator." Proceedings of the Royal Society of London B 269: 1529–1532.