Cucumber (Cucumis sativus L.) is an important crop in Florida and in the US. Cucumbers are grown for the fresh market (slicers) and pickling (processing cucumbers) (Mills 2001), and are grown in the field and in the greenhouse (Larson et al. 2003). In 2013, Florida was the largest producer of slicers in the US and the second largest producer of processing cucumbers (USDA–NASS 2014). In 2013, the value of slicers and processing cucumbers in Florida was $76.3 million and $47.7 million, respectively. Florida also had the highest yield per acre in the US for both cucumber types (USDA–NASS 2014).
Diseases, weeds, and insect pests can adversely affect the yield and quality of cucumbers. In Florida, one of the most common diseases on cucumber is anthracnose, caused by the fungus Colletotrichum orbiculare (syn. C. lagenarium). Anthracnose causes serious economic losses to several economically important vegetable crops worldwide. In addition to cucumber, anthracnose can affect cantaloupe, chayote, citron, gherkin, gourd, honeydew melon, muskmelon, watermelon, and many non-cucurbit species. Anthracnose is rare on pumpkin and squash (Wasilwa et al. 1993). The objective of this publication is to describe the symptoms, causal organism, disease cycle, and management of cucumber anthracnose in Florida.
Symptoms
Anthracnose symptoms occur on all aboveground parts of cucumber plants. Lesions that appear on the cotyledons begin small and water-soaked. They are pale in color, chlorotic (yellow) or necrotic (brown), and are restricted. As the disease progresses the lesions become larger, coalesce, and eventually the cotyledons dry up and die. Similar symptoms appear on the leaves; initial symptoms can have water-soaked appearance and be vein delimited and angular similar to angular leaf spot or downy mildew (Figures 1, 2 and 4). However, as lesions progress they become more rounded, necrotic and often the center of lesions will fall out (Figures 3 and 5). The petioles, fruit pedicel, and stem can also become infected resulting in vine defoliation, fruit decline, and plant death. Fruit can become infected as they begin to mature; fruit lesions appear sunken, circular, water-soaked, and dark in color (Figures 6 and 7). Often the pathogen produces spores, or conidia, on infected fruit giving lesions a wet, pink appearance. Symptoms on the fruit can develop without notice while still in the field and continue to progress after harvest, resulting in infected cucumber fruit in storage and/or transit.

Credit: G. Vallad, UF/IFAS

Credit: G. Vallad, UF/IFAS

Credit: G. Vallad, UF/IFAS

Credit: G. Vallad, UF/IFAS

Credit: G. Vallad, UF/IFAS

Credit: Jason Brock, University of Georgia, Bugwood.org

Credit: Charles Averre, North Carolina State University, Bugwood.org
Causal Organism
When plant tissue samples are submitted to a diagnostic laboratory to confirm cucumber anthracnose, pathogen identification is based on symptoms on the plant and the following description of C. orbiculare conidia. Conidia are the infective structures, or spores, of C. orbiculare. Conidia are formed in masses known as acervuli (singular acervulus), which appear as pink-colored, slimy masses on infected tissue (Zitter et al. 1998) (Figure 8). The acervulus is often surrounded by black, hairlike structures, known as setae (Figure 9) that are only visible under a microscope. The conidia are oval, or pill-shaped, clear, and have no cross walls (Figure 10) (Zitter et al. 1998).


Credit: Paul Bachi, University of Kentucky Research and Education Center, Bugwood.org
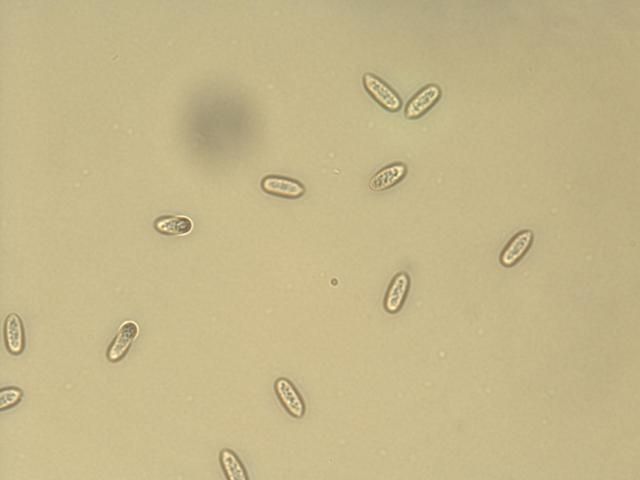
Credit: J. Palenchar and R. Cullen
Many efforts have been made to distinguish C. orbiculare into different races based on host range. Race is a term used to describe a sub-category or group within a fungal species that is genetically and often geographically distinct. Originally, seven races were described that were differentiated based on their ability to infect different cucurbit types (Wasilwa et al. 1993). Recently, two races have been defined based on multiple factors including DNA markers, vegetative compatibility groups, and virulence phenotype. Race 1 is highly virulent on cantaloupe, cucumber, and some watermelon varieties; and Race 2 is moderately virulent on most cucumber varieties and highly virulent on most watermelon varieties (Wasilwa et al. 1993).
Disease Cycle
The fungus C. orbiculare can survive between cucumber crops on seed, volunteer cucurbits, and weeds (such as cockleburs), and on infected crop residues. The spores can be moved by water, workers, and insects, such as Pimelia sp. (Tenebrionidae). Once spores land on susceptible plant parts, they can germinate and form infection structures on the plant known as appressoria. Temperatures of 72–80°F (22–27°C) and a relative humidity of 100% are the optimal conditions for infection to occur. Plant disease symptoms appear about 4 days after infection (Zitter et al. 1998).
Epidemics of anthracnose can reduce yield when they are severe and occur early in the season (Thompson and Jenkins 1985). Temperatures less than 90°F (32°C) and rain will favor disease epidemics. Environmental conditions have a significant influence on the disease progression of anthracnose on cucumber. Anthracnose is less likely to infect cucumber when temperatures get above 86°F (30°C), even if rainfall occurs (Thompson and Jenkins 1985).
Control
An integrated pest management strategy is necessary to effectively manage cucumber anthracnose in Florida. The use of resistant cultivars should be the first step in managing any plant disease and can greatly reduce yield losses due to anthracnose. Several seed companies offer cultivars with varying levels of resistance to this disease, which may be listed as anthracnose, Colletotrichum orbiculare, or "Co" on the seed package, and/or catalogue.
Numerous plant growth-promoting rhizobacteria have been shown to effectively reduce the severity of anthracnose when applied to cucumber seeds before or at planting. This reduction is due to the induction of systemic acquired resistance (SAR) in the cucumber (Raupach and Kloepper 2000; Wei et al. 1991). Systemic acquired resistance is a general resistance response that occurs throughout a plant after the plant experiences injury from a chemical or infection by a pathogen. Cucumber seedlings grown in compost have limited disease severity, which was attributed to SAR-associated gene expression in cucumber plants in greenhouse trials (Zhang et al. 1996). Our recent studies suggest potential for anthracnose suppression by amending potting media with high rates of silicon.
Cultural pest management techniques play a very important role in the prevention and reduction in severity of anthracnose epidemics. Plants should always be started from clean seed (Zitter et al. 1998), and it is desirable to get seed from production areas that are not known to have problems with anthracnose. Cucurbit volunteers and alternative hosts in and around the field and transplant houses should be destroyed. Immediately following the final harvest, deep plowing should be carried out to destroy all infected cucumber plants and debris in the field. Crop rotation with non-hosts for at least 1-year is another management technique that is effective in reducing the incidence of anthracnose (Zitter et al. 1998).
It is very important to monitor or scout cucumber plants for signs of anthracnose, especially when plants are young. Protecting young plants from infection and managing early infections can prevent a build-up of inoculum in the field and can reduce losses (Thompson and Jenkins 1985).
Fungicides are generally effective in controlling anthracnose. Applications of fungicides may need to be applied more often during rainy weather to maintain effectiveness. There are several fungicides labeled for control of cucumber anthracnose in Florida. For conventional producers some of these include chlorothalonil, potassium bicarbonate, copper, pyraclostrobin, mancozeb, azoxystrobin, thiophanate-methyl, and Bacillus subtilis strain QST 713. For organic producers, some allowed fungicides include potassium bicarbonate, coppers, Bacillus subtilis strain QST 713, and some horticultural oils. It is important for both conventional and organic producers to rotate fungicide chemistries to avoid the development of pathogen resistance. Further information is available on application of these and other fungicides for cucumber anthracnose management in the Cucurbit Production Guide chapter of the Vegetable Production Handbook for Florida (https://edis.ifas.ufl.edu/topic_vph). Prior to use of any pesticide, check labels to ensure that the product being applied is labeled for the crop and cropping situation. Always check label for specific recommendations and usage. Read and follow label directions: it is the law!
References
Freeman, Josh H., Eugene J. McAvoy, Peter J. Dittmar, Phil Stansly, Hugh A. Smith, Monica Ozores-Hampton, Mathews Paret, Qingren Wang, Christian F. Miller, and Susan E. Webb. 2016. Curcurbit Production In: Freeman, J., Vallad, G.E., and Dittmar, P. Vegetable Production Handbook for Florida: 2016–17. UF/IFAS, Valent. p. 53–78. https://edis.ifas.ufl.edu/cv123.
Larson, B.C., M.A. Mossler, and O.N. Nesheim. 2003. Florida Crop/Pest Management Profiles: Cucumbers. CIR-1255. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/PI041 (June 2016)
Mills, H.A. 2001. Vegetable Crops: Cucumber, Cucumis spp. University of Georgia, College of Agriculture and Environmental Sciences, Department of Horticulture. http://www.uga.edu/vegetable/home.html (February 2008)
Raupach, G.S., and J.W. Kloepper. 2000. "Biocontrol of Cucumber Diseases in the Field by Plant Growth-Promoting Rhizobacteria With and Without Methyl Bromide Fumigation." Plant Disease 84: 1073–1075.
Thompson, D.C., and S.F. Jenkins. 1985. "Influence of cultivar resistance, initial disease, environment, and fungicide concentration and timing on anthracnose development and yield loss in pickling cucumbers." Phytopathology 75: 1422–1427.
USDA-NASS. 2014. USDA National Agricultural Statistics Service—2014 Agricultural Statistics Annual Report—Vegetables and melons. https://www.nass.usda.gov/Publications/Ag_Statistics/2014/
Wasilwa, L.A., J.C. Correll, T.E. Morelock, and R.E. McNew. 1993. "Reexamination of races of the cucurbit anthracnose pathogen Colletotrichum orbiculare." Phytopathology 83: 1190–1198.
Wei, G., J.W. Kloepper, and S. Tuzan. 1991. "Induction of Systemic Resistance of Cucumber to Colletotrichum orbiculare by Select Strains of Plant Growth-Promoting Rhizobacteria." Phytopathology 81: 1508–1512.
Zhang, W., W.A. Dick, and H. A. J. Hoitink. 1996. "Compost-Induced Systemic Acquired Resistance in Cucumber to Pythium Root Rot and Anthracnose." Phytopathology 86:1066–1070.
Zitter, T.A., D.L. Hopkins, and C.E. Thomas. 1998. "Compendium of Cucurbit Diseases." St. Paul, Minn.: APS Press.