Introduction
Roses are among the most important ornamental flowering shrubs grown worldwide. In the US, the total wholesale production of roses accounts for $194 million per year. Among the major rose producing states in the US, Florida is the fourth largest producer and the production accounts for more than $20 million annually. In Florida, roses have become especially popular in recent years with the introduction of Knock Out® and other shrub roses.
Among the major diseases of roses, rose rosette disease (RRD) is the most destructive. The disease is thought to be caused by the recently identified Rose rosette virus of the genus Emaravirus (Horst and Cloyd 2007; Laney et al. 2011). The disease affects many wild and ornamental rose species and cultivars, so it poses a great concern for the nursery industry and many home gardeners. The disease has been spreading through much of the wild and cultivated rose population in the Midwestern, Southern, and Eastern United States for years. RRD was first observed in 1940 in Manitoba, Canada. In the late 70s and early 80s it was reported as widespread in rural and urban rose plantings in Kansas, Oklahoma, Missouri, and Arkansas. The disease has become widespread in the north-central, south-central, southeast, and mid-west portions of the Northeast and in a few western states in the US (Hong, Hansen, and Day 2012; Windham, Windham, and Hale n.d.). In 2013, RRD was found in Florida (Babu et al. 2014).
Symptoms
Symptoms of RRD are highly variable, depending on the rose species or cultivar affected. Some of the more recognizable symptoms include
- Clustering of small branches (witches'-brooms) (Figure 1)
- Excessive thorn proliferation (Figure 2)
- Unusual reddening of the leaves that does not disappear as the leaf matures (Figure 3)
- Rapid elongation of new shoots (Figure 4)
- Distorted leaf shapes (Figure 5)
- Distorted flower buds (Figure 6)
- Abnormal flower color (Figure 7)
- Leaf mosaic (Figure 8)
- Uneven thickening of stem (Figure 9)
- Distorted sprouting and dieback of shoots (Figure 10)
- Unusual development of leaves within the flower (Figure 11)
- Severe yellowing and stunting of plants (Figure 12)

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Binoy Babu

Credit: Mathews Paret
Infected rose plants often die within one to two years. A diseased plant may exhibit only some of the above mentioned symptoms, especially in the early stages of the disease. Unusual red pigmentation is not a consistent symptom. The new leaves of many rose cultivars normally have reddish pigments, and it may be difficult to determine whether the reddish color is abnormal or not. However, on RRD-infected plants, the reddish color does not go away with age or time, whereas on healthy plants, the reddish color usually disappears as the leaf matures. Therefore, it is important to continue to monitor symptoms on suspect roses. Symptoms of RRD may also resemble herbicide drift damages, which are caused by chemicals such as glyphosate, the active ingredient in Round-up® (Cloyd 2011; Hong, Hansen, and Day 2012; Olson and Rebek 2014). Glyphosate can cause witches'-broom as well as stunted, narrow leaves on roses. The commonly used broadleaf herbicide 2, 4-D can also cause leaf distortion on roses. However, such herbicide injuries should disappear in the following year, unless plants are injured again by spraying. Symptoms of a nutrient deficiency will typically affect the whole plant, whereas appearance of such symptoms on selected parts of the plant are probably due to RRD. Thus, checking for a combination of RRD symptoms is an important part of accurately diagnosing the disease.
History of Rose Rosette Disease
Spread of RRD in the US began with the introduction of the multiflora rose. This exotic plant was introduced from Japan in 1866 as a rootstock for ornamental roses, and it was also used in erosion control, cattle fences, and as a crash barrier on highways. These multiflora roses were capable of producing a million or more seeds per plant and possessed higher vegetative propagation features. These factors ultimately caused the plant to be declared a noxious weed in several states (Hong, Hansen, and Day 2012). Since multiflora roses were highly susceptible to RRD, the disease was initially used as a source of potential biological control against the plant (Armine et al. 1990). However, the disease started gradually spreading from multiflora roses to other cultivated native roses species.
Disease Cycle
Aster yellows phytoplasma was initially thought to cause RRD because it also causes a characteristic witches'-broom–like appearance of affected plants (a phytoplasma is "an organism present in phloem tissue that cannot be grown on artificial media") (Cloyd 2011). Later in 2011, the causal agent of the RRD was found to be a negative-sense RNA virus called Rose rosette virus of the genus Emaravirus (Laney et al. 2011). The disease is transmitted by the eriophyid mite Phyllocoptes fructiphilus and by grafting, but the disease is not sap-transmissible. The wild multiflora rose is highly susceptible to RRD and is one of the most common sources of inoculum for the virus.
Even though the pathogen Rose rosette virus is not soil borne, because of its systemic nature, the virus may persist in infected roots. If such infected root pieces remain in the soil after the removal of infected plants, these remainders could potentially act as a source of infection for the newly planted healthy plants.
Vector
RRD is vectored by the eriophyid mite Phyllocoptes fructiphilus, which is native to North America (Figure 13). These mites are microscopic, measuring 140 to 170 microns long and approximately 50 microns wide. They have a spindle-shaped structure and are typically yellow to brown in color. One of the identifying features of an eriophyid mite is its four legs; other mite species typically have eight legs. Eriophyid mites are typically found in the angles between leaf petioles and axillary buds. In early spring, these mites migrate onto developing shoots, where females lay eggs. Females may live up to 30 days, laying one egg per day. Young mites develop within the leaf folds of new shoots or under leaf petioles. The mites do not possess wings, so they move from plant to plant by attaching to insects and being dispersed by wind (Amrine and Zhao 1998; Hong, Hansen, and Day 2012).
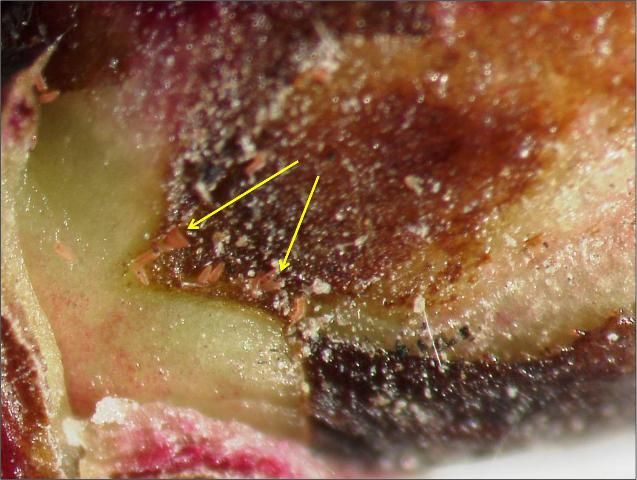
Credit: Baldo Villegas
Management
There is no cure for rose plants that exhibit symptoms of RRD. However, the disease may be prevented from spreading to healthy plants by using a combination of good cultural practices and effective management of mite populations.
Cultural Control
When installing new roses, carefully inspect all plants to ensure they are healthy and free of eriophyid mite and disease symptoms, including symptoms of RRD. Plants should be monitored throughout the season for symptoms of RRD. Infected or symptomatic plants and their roots must be dug up and disposed of immediately. Suspicious plants must be kept separate from healthy plants and monitored for continued symptoms. As soon as RRD is confirmed, infected plants should be removed and destroyed immediately. Diseased plants that have been uprooted should not be allowed to remain in the vicinity of healthy roses because they can continue to serve as a source of infection. It is advisable to remove any multiflora rose plants (frequent sources of inoculum) from the immediate vicinity of rose-growing nurseries and gardens. Planting cultivated roses on hilltops or downwind of known multiflora rose plantings should be avoided because this arrangement will make cultivated rose transplants more susceptible to eriophyid mite invasion (Hong, Hansen, and Day 2012). Ensure that the plants are well spaced so that the canes and leaves do not touch each other; this will prevent the eriophyid mite from moving within a planting.
Chemical Control
One efficient strategy for controlling RRD is using certain miticides to control the eriophyid mite. However, miticides registered for control of spider mites do not necessarily control the eriophyid mites that transmit RRD (Hong, Hansen, and Day 2012). The insecticide Avid® is registered for control of both eriophyid and spider mites on roses (Hong, Hansen, and Day 2012; Star® Roses and Plants/Conard-Pyle 2015). Other potential miticides that are effective against eriophyid mites include bifenthrin (Talstar®), carbaryl (Sevin®), endosulfan (Thionex and Phaser), and petroleum-based horticultural oils (Cloyd 2011). However, these chemicals' EPA labelling needs to be checked with an Extension agent prior to application.
Pruning also removes the mites, which hide near buds and leaf scars. Application of dormant oils on the pruned plants will also help in reducing the remaining mite populations (Olson and Rebek 2014). Fallen debris can also harbor mites, so it is critical to remove and destroy any fallen foliar materials and destroy them before replanting healthy rose plants. Using pesticide alone to control mites is highly risky because the eriophyid mite may develop resistance against the pesticides; therefore, a multi-tactic approach that includes both cultural and chemical practices is the most effective way to manage RRD. Other strategies, such as using predatory mites (Acari: Phytoseiidae) as a biological control agent for eriophyid mites and the development of rose varieties resistant to RRD or mites, may likely control RRD more effectively. These alternative strategies, however, will require further studies.
References
Amrine, J. W., D. F. Hindal, R. Williams, J. Appel, T. Stasny, and A. Kassar. 1990. "Rose rosette as a Biocontrol of Multiflora Rose, 1987–1989," in Proceedings of the 43rd Annual Meeting of the Southern Weed Science Society: 316–320.
Amrine, J. W., and S. Zhao. 1998. "Research on Aerial Dispersal of Phyllocoptes fructiphilus (Acari: Eriophyidae), Vector of Rose Rosette Disease." American Rose 3: 28–29.
Babu, B., H. Dankers, E. Newberry, C. Baker, T. Schubert, G. Knox, and M. L. Paret. 2014. "First Report of Rose rosette virus Associated with Rose Rosette Disease Infecting Knockout Roses in Florida. Plant Disease Notes 98: 1449.
Cloyd, R.A. 2011. Rose Rosette Disease. MF-2974. Kansas State University Plant Pathology Extension, https://bookstore.ksre.ksu.edu/pubs/mf2974.pdf. Accessed September 20, 2022.
Di Bello, P. L, I. E. Tzanetakis, and T. L. Kirkpatrick. Rose Rosette Disease. FSA-7579. University of Arkansas, Division of Agriculture and Natural Resources, Research and Extension, https://www.uaex.uada.edu/publications/pdf/FSA-7579.pdf. Accessed September 20, 2022.
Hong, C., M. A. Hansen, E. Day. 2012. Rose Rosette Disease. 450-620. Virginia State University Cooperative Extension, https://vtechworks.lib.vt.edu/bitstream/handle/10919/48805/450-620_pdf.pdf. Accessed September 20, 2022.
Horst, R. K., and R. A. Cloyd. 2007. Compendium of Rose Diseases and Pests. 2nd ed. St. Paul, MN: APS Press.
Laney, A. G., K. E. Keller, R. R. Martin, and I. E. Tzanetakis. 2011. "A Discovery 70 Years in the Making: Characterization of the Rose Rosette Virus." J Gen Virol. 92: 1727–32.
Olson, J., and E. Rebek. 2014. Rose Rosette Disease. EPP-7329. Oklahoma State University Cooperative Extension Service, https://extension.okstate.edu/fact-sheets/print-publications/epp-entomology-and-plant-pathologhy/rose-rosette-disease-epp-7329.pdf. Accessed September 20, 2022.
Star® Roses and Plants/Conard-Pyle. Rose Rosette Disease: Guidelines for Growers. http://www.southridingnurseries.com/pdfs/seasonal/Rose%20Rosette%20Disease.pdf. Accessed May 18, 2015.
Windham, M., A. Windham, F. Hale. Observations on Rose Rosette Disease. http://www.newenglandgrows.org/pdfs/ho_WindhamRoseRosette.pdf. Accessed May 18, 2015.