Introduction
Leaf nutrient analysis has been widely used as a diagnostic tool to complement soil testing in sugarcane production (Anderson and Bowen 1990; Gascho and Elwali 1979; Samuels 1969). Leaf analysis can be particularly useful in determining the nutrient status of Florida sugarcane because soil samples are routinely only taken before sugarcane is planted and not during ratoon crops because of problems in obtaining representative soil samples after banding of fertilizers (Gascho and Kidder 1979). Also, leaf analysis can provide information about nitrogen, which is not included in standard soil tests in Florida.
Leaf analysis has been used intensively by a limited number of Florida sugarcane growers and has the potential for an expanded role in growers' fertility programs. Leaf analysis evaluation methods and visual symptoms of nutritional problems are described in a companion EDIS publication by McCray et al. (2010b) (https://edis.ifas.ufl.edu/sc075). The purpose of the following document is to provide growers with sufficiency categories of leaf nutrient concentrations and with nutrient management suggestions for each category.
Leaf Nutrient Requirements
Leaf nutrient optimum ranges and critical values are shown in Table 1. The leaf nutrient concentrations defined as the critical value for each nutrient could result in a potential 5%–10% yield reduction from optimum. Also included in Table 1 are nutrient concentrations at which an estimated 25% reduction in final yield may occur. It is important to keep in mind that there are many crop growth factors determining yield, and so other factors will influence the impact of increasing the leaf concentration of a single nutrient. For example, if leaf nitrogen concentration is increased from 1.6 to 2.0%, other nutrients or growth factors may also have to be corrected to get a full 25% yield increase. It is important to examine nutrient sufficiency of all nutrients, as well as to consider the impact of other growth factors such as water availability, drainage, weed control, etc.
Suggested Sample Dates
Comparisons of leaf nutrient concentrations between samples taken in April–May and samples taken in June–August have indicated that leaf manganese and iron concentrations increase with an increase in soil moisture during the summer (McCray et al. 2009). This may occur because of localized areas within the soil of more chemically reduced conditions (oxidation/reduction reactions) in soils that are aerated but with higher soil moisture because of summer rains compared to the normally very dry period of April–early May. Solubility of manganese and iron are each increased as they are reduced to the Mn2+ and Fe2+ ions. This explains the often observed manganese deficiency symptoms in spring that disappear or become less pronounced with the summer rains.
The grand growth period of sugarcane in Florida (June 1 to October 15) is the period of most rapid nutrient uptake (Coale et al. 1993) and so is an appropriate time to evaluate leaf nutrient status. Since this period also generally coincides with the rainy season of late May through October in South Florida, leaf manganese and iron concentrations taken during the grand growth period will generally reflect an increase in soil moisture compared to the normally drier period of April and early May. Soil moisture conditions will obviously vary from year to year and within a given year. Nutrients other than manganese and iron do not appear to be influenced to a large degree by spring versus summer sampling, but sampling leaf tissues early during the grand growth period will work well for evaluating the sufficiency of all nutrients. For these reasons June and July are suggested as preferred months for collecting sugarcane leaf samples in Florida. Samples can also be collected in August and later, but entering fields will be more difficult later in the season. Sample collection and processing is discussed in a companion EDIS publication by McCray et al. (2015) (https://edis.ifas.ufl.edu/sc076).
Sufficiency Categories and Management Strategies
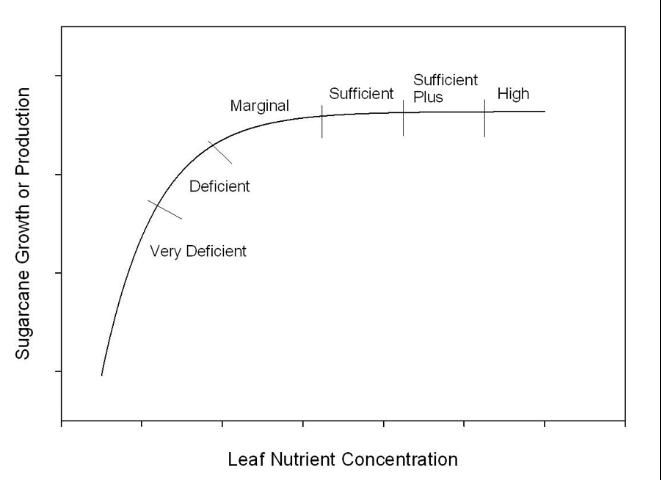
Table 2 lists sufficiency categories for nine nutrients so that the status of each of these can be compared for a given sample/field. Calcium is not included since a true calcium deficiency is rare, and low leaf calcium concentrations are often indicative of other growth or nutritional problems. Table 3 provides basic management strategies for each sufficiency category, and Figure 1 shows a generalized nutrient response curve with the region of each category noted on the curve. The ideal region for all nutrients is the sufficient category because there is no further yield response within this range, and nutrient levels are not in excess. If leaf nutrient concentration is in the sufficient plus or high category, reducing future application rates could be considered, assuming these are nutrients routinely applied in fertilizer. When nutrients are in the deficient or very deficient categories, inclusion of these nutrients in the next application or increasing the planned rate is most likely needed. When a nutrient is in the marginal category, the potential benefit of increasing the concentration to optimum has to be weighed against the cost. It is possible for the maximum economic yield to be slightly down the response curve in the marginal region, depending on the cost/benefit of additional fertilizer. Nitrogen on mineral soils and silicon on organic and mineral soils are examples of nutrients with which cost versus benefit should be closely examined. With these two nutrients it can be difficult to maintain concentrations in the sufficient range, but leaf analysis will help a grower make informed decisions. An effective increase or decrease in rate of a given fertilizer nutrient will depend on soil type, available soil nutrients, and other specifics for a given field. The nutrient sufficiency categories are intended to rank the nutrients in terms of sufficiency and estimate the impact each nutrient is having on yield.
A study of summer fertilizer supplements based on spring leaf analysis indicated that these supplements are too late in the annual growth cycle to result in substantial yield improvements (McCray et al. 2010a). A more cost-effective approach is to use leaf and soil analysis to optimize the next amendment or fertilizer application. This will not require adding unplanned fertilizer applications and will allow for long-term improvements in growers' nutrient management programs. Having leaf nutrient sufficiency categories for a given sample/field will allow a grower to target specific nutrients that are most limiting to sugarcane production.
As part of this approach, two suggested strategies for leaf analysis are:
- To sample representative fields across a farm to assess the fertilizer program each year and
- To sample fields in the last ratoon year before replanting to assist in making amendment decisions (calcium silicate, dolomite, and elemental sulfur).
This plan would not require sampling every field, every year, and should provide a reasonable annual picture of nutrition for a farm. Leaf analysis can provide a relatively inexpensive source of nutritional information to supplement soil testing for making improved nutrient management decisions.
References
Anderson, D. L. and J. E. Bowen. 1990. Sugarcane nutrition. Potash & Phosphate Institute, Atlanta, GA.
Coale, F. J., C. A. Sanchez, F. T. Izuno, and A. B. Bottcher. 1993. "Nutrient accumulation and removal by sugarcane grown on Everglades Histosols." Agron. J. 85:310–315. https://doi.org/10.2134/agronj1993.00021962008500020028x
Gascho, G. J. and A. M. O. Elwali. 1979. "Tissue testing of Florida sugarcane." Sugar J. 42:15–16.
Gascho, G. J. and G. Kidder. 1979. Responses to phosphorus and potassium and fertilizer recommendations for sugarcane in south Florida. Bulletin 809. Gainesville: University of Florida Institute of Food and Agricultural Sciences.
McCray, J. M., R. W. Rice, and I. V. Ezenwa. 2015. Sugarcane leaf tissue sample preparation for diagnostic analysis. SS-AGR-259. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/sc076
McCray, J. M., S. Ji, G. Powell, G. Montes, R. Perdomo, and Y. Luo. 2009. "Seasonal concentrations of leaf nutrients in Florida sugarcane." Sugar Cane International 27:17–24.
McCray, J. M., S. Ji, G. Powell, G. Montes, and R. Perdomo. 2010a. "Sugarcane response to DRIS-based fertilizer supplements in Florida." J. Agron. Crop Sci. 196:66–75. https://doi.org/10.1111/j.1439-037X.2009.00395.x
McCray, J. M., R. W. Rice, T. A. Lang, and Les Baucum. 2010b. Sugarcane plant nutrient diagnosis. SS-AGR-128. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/sc075
Samuels, G. 1969. Foliar diagnosis for sugarcane. Adams Press, Chicago.
Sugarcane leaf nutrient concentration optimum ranges and concentrations at which a 5–10% or 25% production loss from optimum might be expected.†