Introduction

Credit: Lyn Gettys, UF/IFAS
Hydrilla (Hydrilla verticillata), which was originally introduced to Florida as an aquarium plant, was intentionally planted in canals by aquarium plant dealers in the 1950s and quickly escaped cultivation. Hydrilla is a submersed aquatic plant that grows throughout the year in most areas of Florida but undergoes winter dieback in northern parts of the state and in more temperate regions of the United States. More public funds are spent managing hydrilla than any other aquatic plant in Florida's waters. For example, between 2011 and 2022, aquatic resource managers in Florida spent $167.5 million in state and federal dollars for aquatic plant management, and over half of those funds were used for hydrilla management (FWC 2024). Hydrilla is a Florida Noxious Weed, which means that transportation, importation, collection, possession, cultivation, or sale of the species is illegal in the state. These activities are also prohibited throughout the United States because hydrilla is on the Federal Noxious Weeds List (USDA APHIS 2010). Nevertheless, the species is still occasionally available for purchase from certain farmers' markets, yard sales, aquarium supply stores, aquatic plant nurseries, and Internet sources in other states.
There is only a single species of hydrilla, but there are two distinct biotypes—monoecious (producing separate "male" and "female" flowers on the same plant) and dioecious (each plant producing only "male" or "female" flowers). Hydrilla in Florida is of the dioecious type and produces only "female" flowers; there are no pollen-producing "male" plants in the state and, as a result, no means for seed production. Monoecious hydrilla is thought to be native to Korea, whereas the dioecious biotype is considered a southern Indian native (Madeira et al. 1997).
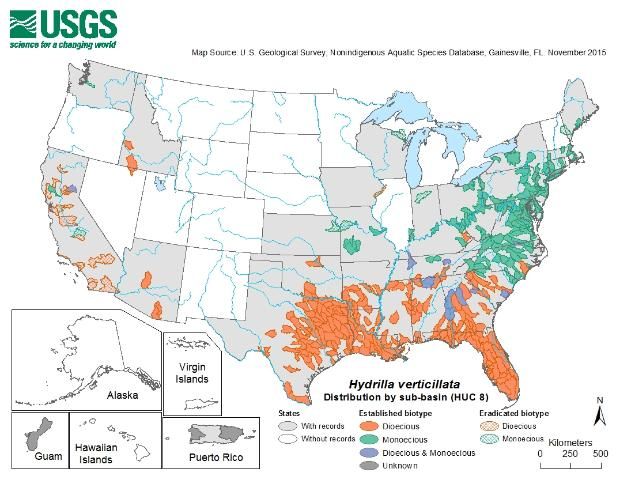
Credit: United States Geological Survey
Both biotypes can be found in North America. The dioecious biotype is prevalent throughout Florida and the Gulf states south of Tennessee. It was also found in Arizona, but those populations have since been eradicated. Dioecious hydrilla also occurs in the warm geothermal springs of Idaho, but this biotype occurs primarily in the southern US. The monoecious biotype of hydrilla is broadly distributed throughout the watersheds of the mid-Atlantic and northeastern United States. This biotype has reportedly been eradicated from the small urban or farm ponds where it was previously found in Wisconsin, Washington state, and Iowa. Eradication efforts are currently underway in Massachusetts, Maine, and New York.
Dioecious hydrilla is a perennial, but monoecious hydrilla behaves more like an annual. This is especially true in northern states, where monoecious hydrilla dies back to the ground during the winter and resprouts from overwintering structures such as tubers and turions the following spring (Langeland 1996). The two biotypes of hydrilla are very similar in appearance, making genetic analyses necessary to differentiate between them. Regardless of biotype and nativity, hydrilla is considered one of the world's worst weeds (Velez-Gavilan 2022). It has invaded ecosystems around the globe. The first sightings of hydrilla in the United States occurred in 1960 at two Florida locations, which were a canal near Miami and springs near Crystal River (Blackburn et al. 1969). The plants were initially misidentified as the native species Elodea canadensis, and they quickly established a foothold in the state. Hydrilla had invaded more than 35,000 acres of water in Florida by 1967 and was considered a serious aquatic weed. Researchers noticed its unusual growth and apparent tolerance of aquatic herbicides and sent samples of the plants to Dr. Harold St. John, an authority on Elodea taxonomy and identification, who determined that the mystery plant was Hydrilla verticillata. The species was intentionally planted by aquatic plant dealers on Florida's west coast in 1958 and in the southeast part of the state in 1959 (Blackburn et al. 1969). It seems likely that these plantings were the source populations for the initial infestations in 1960.
Classification
Common Names: hydrilla, waterthyme
Scientific Name: Hydrilla verticillata (L. f.) Royle
Family: Hydrocharitaceae (tape-grass or frog's-bit family)
Description and Habitat

Credit: Lyn Gettys, UF/IFAS
Hydrilla is an obligate submersed plant that is rooted in the substrate and grows completely underwater. It can survive in nutrient-rich or nutrient-poor water at depths from a few inches to 25 feet, but it cannot survive out of the water. The leaves of hydrilla are green and straplike with pointed tips and serrate (saw-toothed) margins. The midrib or central vein on the underside of the leaf may have one or more sharp teeth as well. Part of hydrilla's scientific name is derived from the plant's leaf arrangement—leaves are arranged around the stem in a verticillate (whorled) manner, with four to eight leaves attached at each node. The long, slender, and branching stems can be up to 25 feet long and quickly fill the water column with nearly impenetrable growth that hinders water flow, navigation, and recreational use of the water. When hydrilla reaches the surface, it forms dense mats that block the penetration of light and oxygen into the water column.
Florida populations of hydrilla produce tiny white flowers measuring 0.0625 to 0.125 inches in diameter. These flowers are borne on threadlike peduncles (flower stalks) that are up to 4 inches long. Like other water-pollinated members of the Hydrocharitaceae family, the "female" flowers of hydrilla float on the surface of the water to facilitate pollination by free-floating "male" pollen-bearing flowers. However, "male" flowers are not produced by any known population of hydrilla in Florida, so pollination and seed production are not possible. Instead, hydrilla reproduces and spreads primarily through fragmentation. Even small stem sections can form roots and develop into independent plants that increase the range of this noxious weed. Hydrilla also produces two specialized structures which allow it to colonize new areas and survive harsh conditions created by herbicide treatments, drought, and freezing temperatures. Turions are small (up to 0.25 inches long), dark-green structures that are borne in the leaf axils and resemble tiny pine cones. Tubers are slightly larger (up to 0.5 inches long and wide), white to yellow, subterranean structures that look like small peeled potatoes and are produced on rhizomes in the substrate. Turions and tubers allow hydrilla to regrow after winter freezes and other harsh conditions. Tubers are viable for many years. A single tuber, when allowed to sprout and grow into a plant, can ultimately yield around 700 progeny tubers per square foot (UF/IFAS CAIP 2024).

Credit: Lyn Gettys, UF/IFAS
It is easy to confuse hydrilla with other submersed plants because hydrilla's appearance is similar to that of other aquatic plants, including native elodea (Elodea canadensis) and exotic Brazilian waterweed (Egeria densa). However, differences among several characteristics can be used to correctly distinguish these look-alike species. For example, all three species have leaves attached in whorls, but hydrilla leaves have distinctly serrate margins. The margins of elodea leaves are smooth, and those of Brazilian waterweed leaves have minute teeth. All have white flowers, but the flowers vary in size. Hydrilla flowers are tiny, elodea flowers are slightly larger, and Brazilian waterweed flowers are the largest. Hydrilla has the broadest distribution of the three species in Florida and can be found in many bodies of water from the extreme south to the extreme north. Elodea is considered rare in Florida and has only been reported in Jackson County, which borders Alabama and Georgia (USDA NRCS 2024), while Brazilian waterweed is somewhat common in central and north Florida and rare in south Florida. A defining characteristic of hydrilla is the ability to produce turions and tubers. No other submersed species produces these structures. The plant in question can be positively identified as hydrilla if it has these turions and tubers.
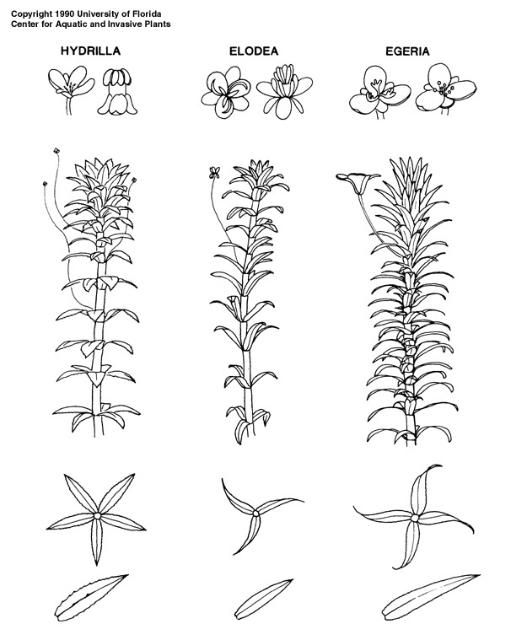
Credit: UF/IFAS CAIP
The prolific growth of hydrilla has caused business-minded individuals to wonder whether the biomass produced by the species could be exploited for profit. There are numerous websites that offer powdered hydrilla (either loose or in capsules) as a dietary supplement. Hydrilla has been used in India as fish food, storage and packing material for live crabs, and an organic amendment for crop production (Misra et al. 2012). The species has also been evaluated as a biogas source (due to its high apparent biomass production) as well as a phytoremediation agent for nutrient-rich waters (Evans and Wilkie 2010). These reports suggest that hydrilla may be useful under certain circumstances, but the plant's prodigious growth leads to environmental damage that far outweighs any possible benefits. Hydrilla and other submersed plants are considered shade plants by ecologists because they grow under low light conditions and produce less true biomass than full-sun terrestrial species do. Although dense hydrilla populations can produce 20,000 to 30,000 pounds of wet biomass per acre during a season, their biomass is 95% water; the true dry biomass produced is actually less than 1 ton per acre. In comparison, sugarcane and other row crops produce as much as 10 to 20 tons per acre of dry biomass; therefore, hydrilla has little potential as a biofuel or biogas source.

Credit: Lyn Gettys, UF/IFAS
Hydrilla grows very quickly. For many years, it was believed that the species could grow an inch per day. However, Glomski and Netherland (2012) found that a single 5-inch long cutting of hydrilla rooted, grew, and expanded three-dimensionally by forming hundreds of new stems, lateral branches, and stolons over the course of a 5-week period. Collectively, new growth produced by the original cutting represented lengthening of up to 191 inches per day (Glomski and Netherland 2012), so the "inch per day" theory is a drastically low estimate of hydrilla's growth potential. Due to its robust growth and expansion, hydrilla routinely outcompetes native aquatic plants and forms monocultures (populations of a single species) that are poor habitats for aquatic fauna, most of which require a diverse environment in order to thrive. Dense plant growth and surface mats also trap heat, which raises the temperature of surface water and depletes dissolved oxygen. Hydrilla obstructs water flow in flood-control canals, which can make it difficult or impossible for resource managers to move large volumes of water during tropical storms, hurricanes, and other severe weather events. Hydrilla also negatively affects recreational uses of infested waters. Boat motors quickly become clogged with weeds, fishing lines may snag soon after being cast, and swimmers have reportedly drowned after becoming entangled in dense hydrilla beds (Joyner 2011; Robbins 2015; Shelly 1990).
Control
The best way to control hydrilla is through prevention or exclusion from aquatic ecosystems. Accidental transfer of hydrilla and other submersed species frequently occurs when boats, trailers, and other equipment are moved from an invaded site to a pristine one. Therefore, any object that has been launched into waters where hydrilla is present should be carefully inspected and cleaned before being moved to another body of water to ensure that any plant fragments clinging to the vessel or equipment are removed (Gettys et al. 2013). Once hydrilla becomes established, there are a number of methods that can be used to manage this noxious weed.

Credit: Bill Haller, UF/IFAS
Benthic barriers are geotextiles that are installed over nascent, low-growing populations of hydrilla to block light penetration. Unfortunately, these can be expensive and require regular maintenance to be effective. Drawdowns can be used to desiccate and destroy hydrilla shoots filling the water column, but they require long-term dewatering and can be impossible or impractical, because tubers and turions survive drawdowns and sprout to form new hydrilla plants once the water is restored to its normal level. Mechanical control is the removal of plant material, usually with specially designed equipment. Historically, aquatic harvesters were limited to operating in the upper 5 feet of the water column. Harvesting could only be used to "cut the grass" on hydrilla populations that were near the surface of the water and had to be repeated throughout the season to keep weeds in check. Also, bycatch, or the unintentional capture of fish and other fauna along with hydrilla, was a significant problem. Fish living in waters with dense hydrilla infestations tend to stay near the surface of the water in order to gain easier access to oxygen. Newer aquatic harvesters can operate in the upper 10 feet of the water column. As a result, populations can be trimmed while they are still low-growing, and longer periods between harvests are possible. Deepwater harvesting performed on lower-density populations reduces problems related to dissolved oxygen availability, helps keep fish in deeper strata of the water, and decreases bycatch (Haller 2020).

Credit: Bill Haller, UF/IFAS
Biological Control
The introduction of an invader's natural enemies may be a useful tool for hydrilla management. Biological control agents, also called biocontrol agents, are either generalist (prone to attacking many species) or host-specific (only attacking a single species). The generalist grass carp (Ctenopharyngodon idella), also known as the white amur, is used for hydrilla management in more than 50 countries and was introduced to Florida in 1970 to evaluate its potential as a hydrilla biocontrol agent. The grass carp's status as an introduced exotic species raised concerns that the fish could escape cultivation and establish reproducing populations in Florida's waters. This problem was addressed when sterile triploid grass carp were developed in the 1980s. Triploid grass carp are sterile because they possess an extra set of chromosomes. This condition is induced by subjecting fertilized eggs to a shock such as heat, cold, or pressure, which renders them unable to reproduce (Hoyer 2020). These fish are voracious consumers of hydrilla, but they are also generalists that will feed on a wide variety of aquatic species, including desirable native plants. The possession of triploid carp is regulated, and a permit must be obtained from the Florida Fish and Wildlife Conservation Commission in order to introduce them to Florida's waters (Hoyer 2020). There are a number of other generalist herbivores that feed on hydrilla, including the hydrilla leafcutter moth (Parapoynx diminutalis). The leafcutter moth was evaluated as a potential biocontrol agent for hydrilla in 1971 but was not introduced to the US because it is a generalist herbivore. However, the moth was discovered feeding on a Florida population of hydrilla in 1976. The hydrilla leafcutter moth's introduction pathway is unknown, but it is now widely distributed throughout Florida. The moth also consumes a wide variety of both native and exotic aquatic plants (Baniszewski et al. 2023).
There are a number of host-specific biocontrol agents that cause varying levels of damage to hydrilla. Some, such as tip mining midges, stem weevils, and leaf mining flies, attack vegetative parts of the plant, while tuber weevils cause damage to its subterranean tubers (Cuda et al. 2022; Weeks et al. 2024; Weeks and Cuda 2022; Weeks 2022). These insects do not provide a sufficient level of hydrilla control on their own, but they can be useful when used in conjunction with other control methods as part of an integrated pest management (IPM) program.
The primary means for managing hydrilla is chemical control, or the use of herbicides. Before being labeled for aquatic use, herbicides are evaluated by the United States Environmental Protection Agency as well as the state of Florida. An herbicide is granted a label only if these agencies determine that it will perform its intended function without unreasonable adverse effects on human health or the environment when applied according to label instructions (USEPA 2012). Label instructions must be followed to ensure that a particular herbicide is applied in the proper manner. Failure to comply with the label instructions is a violation of federal law. Accidental or intentional misuse of a pesticide is a serious offense that may result in significant fines and imprisonment (Fishel 2019). For more information and details about hydrilla management options and control measures, please see the EDIS publication, Hydrilla Management in Florida Lakes, at https://edis.ifas.ufl.edu/publication/ag370.
For greatest efficacy and most responsible stewardship, the tools used for the management of hydrilla and other weeds should be used in an integrated manner. For example, the use of biocontrol insects along with herbicides can result in better control than the exclusive use of either tool. Herbicides with different active ingredients should be rotated to reduce the likelihood of weed populations developing resistance. For more information about how and why aquatic weeds are managed in Florida, please visit Plant Management in Florida Waters, a website developed by the UF/IFAS Center for Aquatic and Invasive Plants and the Florida Fish and Wildlife Conservation Commission, at https://plants.ifas.ufl.edu/manage/.
Summary
Hydrilla, which was originally introduced to the state as an aquarium plant, was intentionally planted in canals by aquarium plant dealers in the 1950s and quickly escaped cultivation. In addition to being one of the world's worst aquatic weeds, the species is Florida's most intensively managed submersed plant. Hydrilla is a federally listed noxious weed and a prohibited aquatic plant in Florida, making cultivation, sale, and possession of the species illegal. Hydrilla quickly clogs the water column and makes it difficult or impossible to move storm waters. Recreational activities and ecosystem services are disrupted by hydrilla as well. There are a number of control methods that may be used to manage hydrilla populations, but aggressive maintenance control programs that rely on the use of herbicides are most commonly used to keep populations of hydrilla at tolerable levels.
References
Baniszewski, J., E. N. I. Weeks, and J. P. Cuda. 2023. Hydrilla Leafcutter Moth (Unofficial Common Name): Parapoynx diminutalis Snellen (Insecta: Lepidoptera: Crambidae). IN1024. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/in1024
Blackburn, R. D., L. W. Weldon, R. R. Yeo, and T. M. Taylor. 1969. "Identification and distribution of certain similar-appearing submersed aquatic weeds in Florida." Hyacinth Control Journal 8: 17–23.
Velez-Gavilan, J. 2022. “Hydrilla verticillata”. In CABI Compendium Issue 170: Compendia Datasheets. Wallingford, UK: CAB International. Accessed July 10, 2024. https://www.cabidigitallibrary.org/doi/epdf/10.1079/cabicompendium.28170
Cuda, J. P., B. R. Coon, E. N. I. Weeks, J. L. Gillmore, and T. D. Center. 2022. Hydrilla Tip Mining Midge: Cricotopus lebetis Sublette (Insecta: Diptera: Chironomidae). IN211. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/in211
Evans, J. M., and A. C. Wilkie. 2010. "Life cycle assessment of nutrient remediation and bioenergy production potential from the harvest of hydrilla (Hydrilla verticillata)." Journal of Environmental Management 91(12): 2626–2631. doi: 10.1016/j.jenvman.2010.07.040
Fishel, F. M. 2019. Federal Regulations Affecting Use of Pesticides. PI168. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/pi168
FWC. 2024. “Annual Reports: Plant Control by Plant Species and Waterbody”. Aquatic Plant Management. Accessed July 10, 2024. https://myfwc.com/wildlifehabitats/habitat/invasive-plants/aquatic-plant/
Gettys, L. A., W. T. Haller, and G. E. MacDonald. 2013. "Chapter 13: Herbicides in aquatic systems." In Herbicides—Current Research and Case Studies in Use, ed. A. Price, 329–351. https://doi.org/10.5772/56015
Glomski, L. M., and M. D. Netherland. 2012. "Does hydrilla grow an inch per day? Measuring short-term changes in shoot length to describe invasive potential." Journal of Aquatic Plant Management 50: 54–57.
Haller, W. T. 2020. "Chapter 3.5: Mechanical control of aquatic weeds." In Biology and Control of Aquatic Plants, A Best Management Practices Handbook: Fourth Edition, ed. L. A. Gettys, W. T. Haller, and D. G. Petty, 133–138. Marietta: Aquatic Ecosystem Restoration Foundation.
Hoyer, M. 2020. " 3.6.2 Grass Carp for Biocontrol of Aquatic Weeds." In Biology and Control of Aquatic Plants, A Best Management Practices Handbook: Fourth Edition, ed. L. A. Gettys, W. T. Haller, and D. G. Petty, 151–154. Marietta: Aquatic Ecosystem Restoration Foundation.
Joyner, T. 2011. "Family of drowned teen wants more safeguards at Clayton County International Park." Atlanta Journal-Constitution, January 21, 2011. Accessed July 10, 2024. https://www.ajc.com/news/local/family-drowned-teen-wants-more-safeguards-clayton-county-international-park/wkIip6RXthnKtRrXeJ9CYM/
Langeland, K. A. 1996. "Hydrilla verticillata (L. f.) Royle (Hydrocharitaceae), 'The perfect aquatic weed.'" Castanea 61(3): 293–304.
Madeira, P., T. Van, K. Steward, and R. Schnell. 1997. "Random amplified polymorphic DNA analysis of the phenetic relationships among world-wide accessions of Hydrilla verticillata." Aquatic Botany 59: 217–236.
Misra, M. K., A. Panda, and D. Sahu. 2012. "Survey of useful wetland plants of south Odisha, India." Indian Journal of Traditional Knowledge 11(4): 658–666.
Robbins, S. 2015. "Drownings of immigrants crossing Rio Grande rise." Charleston Daily Mail, March 29, 2015. Accessed July 10, 2024. https://www.wvgazettemail.com/news/drownings-of-immigrants-crossing-rio-grande-rise/article_ecba8c48-1f3e-5042-948d-4907e9212893.html
Shelley, J. 1990. "Hydrilla plant poses danger for Lake Osborne swimmers." Sun-Sentinel (Broward County), June 20, 1990. Accessed July 10, 2024. https://www.sun-sentinel.com/news/fl-xpm-1990-06-20-9001140657-story.html
UF IFAS CAIP. 2024. "Hydrilla: Hydrilla verticillata." University of Florida IFAS Center for Aquatic and Invasive Plants. Accessed July 10, 2024. https://plant-directory.ifas.ufl.edu/plant-directory/hydrilla-verticillata/
USDA APHIS. 2010. "Federal Noxious Weed List." United States Department of Agriculture Animal and Plant Health Inspection Service. Accessed July 10, 2024. https://www.aphis.usda.gov/sites/default/files/weedlist.pdf
USDA NRCS. 2024. "Elodea canadensis Michx.: Canadian waterweed." United States Department of Agriculture Natural Resources Conservation Service. Accessed July 10, 2024. https://plants.usda.gov/home/plantProfile?symbol=ELCA7
USEPA. 2012. "Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA)." Accessed July 10, 2024. https://www.agriculture.senate.gov/imo/media/doc/FIFRA.pdf
Weeks, E. 2022. Hydrilla Tuber Weevil: Bagous affinis Hustache (Insecta: Coleoptera: Curculionidae). IN1039. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/in1039
Weeks, E., and J. Cuda. 2022. Hydrilla Leaf Mining Flies (Unofficial Common Name): Hydrellia spp. (Insecta: Diptera: Ephydridae). IN1034. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/in1034
Weeks, E., J. Cuda, and M. J. Grodowitz. 2024. Hydrilla Stem Weevil: Bagous hydrillae O'Brien. IN1036. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/in1036