Nitrogen (N) is an essential nutrient for plant growth and crop production. Cropping systems are costly operations, so implementation of best management practices for N fertilization is crucial to increase efficiency and profitability. The N cycle involves a series of linked natural processes involved in the movement of N among different pools of the air-soil-plant-animal interface of croplands and grasslands (Figure 1) that can be difficult to define. However, understanding the basics of the N cycle helps improve the use of fertilizers (organic or inorganic) to meet crop needs while safeguarding the environment. The N can be lost from soils that produce grain and hay crops through several processes, including volatilization, soil erosion and runoff, leaching, and denitrification. However, implementation of proper management techniques can mitigate these losses of N from cropping lands. This publication summarizes N cycling, different types of N losses, and approaches that may help to minimize N loss in row crop production systems in Florida. The information in this document may be of interest to Extension agents and row crop producers.
Nitrogen Cycle
The N cycle (Figure 1) illustrates the processes through which N is converted into different forms, consecutively passing from the atmosphere to soil to living organisms and back to the atmosphere. Figure 1 shows the pathways of N from manure, fertilizers, and plants through the soil to crops, water, and the air. The red text represents losses of N, the blue text represents forms of N, and the black text describes processes in N cycling.
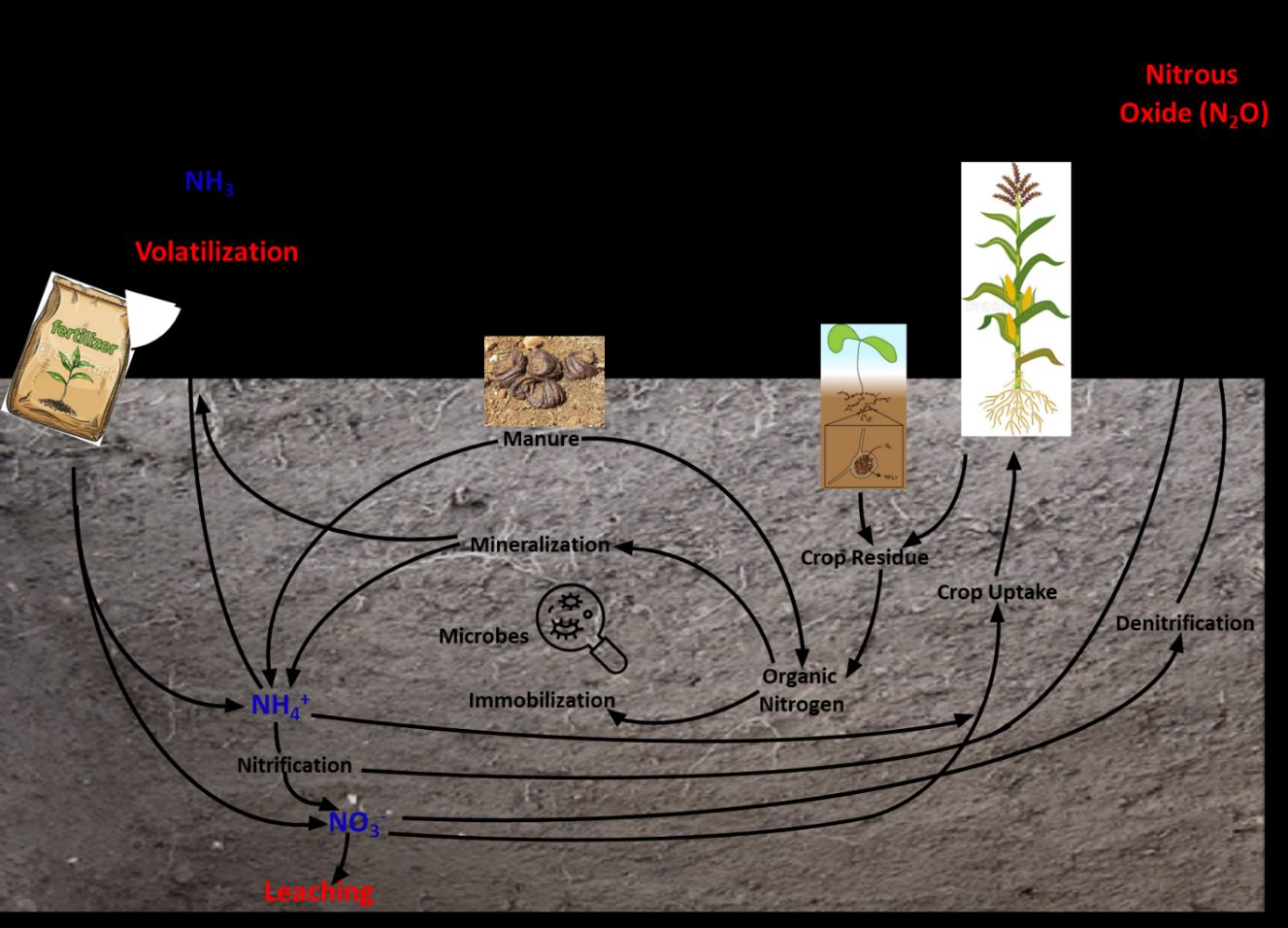
Credit: Hardeep Singh, UF/IFAS
Sources of Nitrogen
There are different sources of N available in cropping systems.
- Inorganic fertilizers: The fertilizer industry fixes atmospheric N (N2) through different chemical processes known as the Haber-Bosch process.
- Conventional (e.g., urea)
- Enhanced efficiency fertilizers (e.g., controlled release, slow-release, urease, nitrification inhibitors, etc.)
- Organic amendments:
- Plant residues (e.g., leftover main crop residue or cover crop)
- Animal manures (e.g., chicken litter)
- Composts
- Biosolids
- Dinitrogen (N2) gas in the atmosphere can be fixed into plant-available N forms by specialized microbes that are free-living in the soil or in root nodules found on legume roots. This is known as biological N fixation.
- There are also small annual N inputs in rainfall, snowmelt, and dust.
The primary forms of N found in N fertilizers are ammonium (NH4), nitrate (NO3), and urea (CO(NH2)2), or combinations thereof.
Nitrogen Reactions in Soil
After N sources enter the soil, all forms of N undergo various chemical changes through multiple processes. These changes involve mineralization, nitrification, and denitrification. Mineralization is the transformation of N present in organic form in the soil into plant-available N (ammonium). Nitrification is a process by which ammonium is converted into nitrate by the action of soil microbes. Under natural conditions, nitrification typically occurs days after N source application. Denitrification is the conversion of nitrate to nitrous oxide (N2O) or N2 by a microbial population under anaerobic conditions with an organic carbon source.
Losses of Nitrogen and Conducive Conditions
Nitrogen can be lost from soils that produce grain and hay crops through several processes, including volatilization, runoff, leaching, and denitrification.
Volatilization
Volatilization can be explained as the loss of the ammonium (NH4) form of N from surface-applied fertilizers to the atmosphere as ammonia (NH3) gas. It is the most common N loss in upland soils under rainfed conditions. In particular, the ammonium-forming fertilizers, such as urea (46–0–0) and urea ammonium nitrate (UAN, 28/32–0–0), are prone to volatilization when left on the soil surface. Most row crop producers in Florida use urea as a source of N and broadcast it, which results in conditions conducive to volatilization losses. Additionally, warmer temperatures, greater soil moisture, and windy conditions favor N volatilization losses in row crop production systems.
Runoff
Runoff can be defined as lateral movement of N beyond the root zone due to the heavy flow of water. Nitrogen lost via runoff may be influenced by topography, soil type, soil compaction, soil moisture, rainfall, and fertilizer type. After heavy rain, surface runoff water may carry N away from surface-applied fertilizer or stored manure, thereby impacting nearby water bodies. While Florida receives ample rainfall, the loss of N as runoff is very low due to predominantly sandy soils with greater water infiltration capacity, which allows vertical water movement in soils rather than lateral movement. However, runoff can still play a role in N losses in sloping areas.
Leaching
The vertical movement of soluble N below the rooting zone is called leaching. Compared to heavier soils, the sandy soils in Florida have greater water infiltration capacity, allowing vertical movement of water rather than lateral movement. Therefore, heavy rainfall patterns and greater infiltration capacity of Florida soils result in more N losses via leaching rather than runoff for row crop production systems. Excess water moving downward to recharge groundwater can carry nitrates from soil, resulting in nitrate accumulation in groundwater. This is a major concern, as it degrades groundwater quality. The management practices that minimize water movement beyond the crop root zone could help lower N losses from leaching.
Denitrification
Denitrification is the conversion of nitrate into nitrous oxide or dinitrogen by soil microbes when the water-filled pore space of the soil is greater than 70%, favoring anaerobic conditions. Additionally, denitrification requires organic carbon, which supplies energy to denitrifying microorganisms. Denitrification results in nitrous oxide (N2O), a potent greenhouse gas, or N2; both are lost to the atmosphere. It is more common in heavier soils coupled with high rainfall and slow crop growth. It is the most common N loss in lowland soils, but it also occurs in upland soils if N is applied before large rainfall events. Additionally, denitrification can result in significant N losses in flatwoods soils with spodic horizon. Impaired drainage can result in N losses in coarse-textured soils. Depending on soil properties and environmental conditions, approximately 5%–25% of the total N applied can be lost via denitrification (Adotey et al. 2021).
Approaches to Reduce Nitrogen Losses
Leaching and volatilization are the significant N fertilizer losses in Florida row crop production systems. The best management practices that lower the leaching and volatilization losses are discussed below.
Implementing 4R Stewardship
Implementation of 4R stewardship helps optimize applied N fertilizer, maximize plant uptake, and minimize environmental losses. The 4R’s are right source, right rate, right time, and right place. Right source refers to selection of fertilizer that delivers adequate amounts of the nutrient in the form the plant needs. Right rate refers to delivering adequate amounts of the nutrient that the plant needs, because over-application results in environmental losses and under-application results in production losses. Right time refers to application of fertilizer when the plant needs it, which depends on the growth pattern of the crop. Lastly, right place refers to placing the fertilizer where the plant can easily access the nutrient, which depends on the spread of the root system. Different placement methods include broadcasting, banding, or subsurface banding, which are discussed in the following subsection.
Broadcasting vs. Subsurface Banding
Broadcasting refers to spreading the fertilizer evenly over the soil surface, which may or may not be incorporated using primary or light tillage. Banding refers to placing the fertilizer close to the crop roots, about 4 to 6 inches below the soil surface. Subsurface banding of N fertilizers helps in reducing volatilization losses. Previous research findings confirm that banding urea reduced NH3 volatilization losses by about 20% compared to surface application. Even though subsurface band application requires specific equipment and may increase expenses, the savings associated with reduced N fertilizer use might help in offsetting the costs. Another potential issue associated with band application includes excess salinity that could affect seedling development, depending on the type of fertilizer used. Therefore, using subsurface band application of N fertilizers for row crop production systems in Florida helps reduce volatilization losses, increase crop uptake, and increase N use efficiency. Figure 2 demonstrates broadcasting and subsurface band application of N fertilizers in row crop production systems in Florida.

Credit: Hardeep Singh, UF/IFAS
Use of Enhanced Efficiency Nitrogen Fertilizers
Enhanced efficiency nitrogen fertilizers (EENFs) are urea or UAN fertilizers that are chemically treated or physically coated to facilitate slow release of N and decrease volatilization, runoff, leaching, or denitrification losses. EENFs can be grouped into two categories: stabilized N fertilizers and slow-release N fertilizers.
For the stabilized N fertilizers, the stability of certain N forms is increased using one or more chemicals, including urease and nitrification inhibitors. N-(n-butyl) thiophosphoric triamide (NBPT) is considered an effective urease inhibitor in different cropping systems, inhibiting urease enzyme activity through competitive inhibition, and slowing down the conversion of urea to NH3. This inhibitor reduces urease activity for 7–14 days (Figure 3). NBPT-treated urea is reported to have about 52% lower ammonia losses than untreated urea (Cantarella et al. 2018). Therefore, the use of urease inhibitor, namely NBPT, can be a potential management practice for lowering N losses from urea and UAN. The mode of action for nitrification inhibitors is different because they slow down the activity of soil microbes responsible for converting ammonium to nitrate form, and they prevent leaching and denitrification losses. The most commonly available nitrification inhibitors are 2-chloro-6-(trichloromethyl)-pyridine (nitrapyrin), and dicyandiamide (DCD). Another form of EENF is controlled-release N fertilizers, where the conventional fertilizers are physically coated using water-insoluble, permeable, or semipermeable material (such as polymer, resin, etc.), and help to reduce volatilization, leaching, and denitrification N losses. Common slow-release N fertilizers include neem-coated urea and environmentally smart nitrogen (ESN). Table 1 lists some available EENFs along with their recommended application rates.
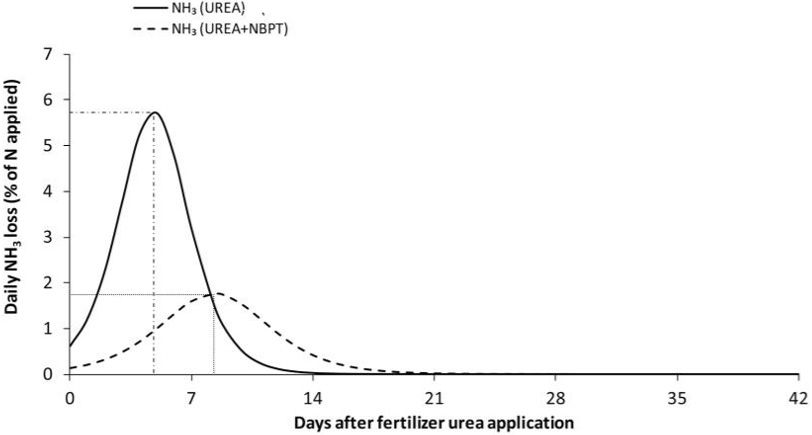
Credit: Adapted from Silva et al. (2017)
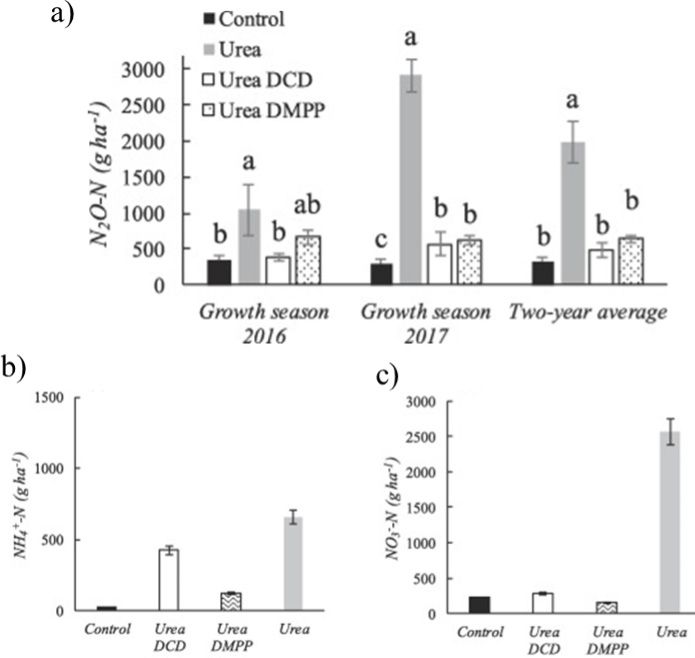
Credit: Adapted from Meng et al. (2021)
Table 1. Product names, manufacturers, active ingredient concentrations, and recommended rates of different commercially available EENFs.
Improving Soil Health
Due to the warm climate and sandy soils in Florida, the organic matter in the soil is relatively low. Increasing the soil organic matter helps in improving soil cation exchange capacity and nutrient retention capacity. Row crop producers can increase soil organic matter by reducing soil disturbance, adopting conservation tillage practices (e.g., strip-tillage), utilizing cover crops in crop rotations, and incorporating organic amendments. Using cover crops is one effective technique for improving soil health. The practice provides several other benefits, including reduced erosion, N credits associated with legume use (although they depend on several other factors), and weed suppression. Including winter cover crops in the rotation would help increase soil organic matter. Non-legume winter cover crops would help scavenge the leftover N applied to row crops and reduce N leaching during winter.
Additionally, the N scavenged by cover crops during winter can be made available to the following crop during decomposition. Long-term use of these practices helps to increase nutrient recycling, diversity, and abundance of soil microorganisms, and to lower bulk density, which helps root development. Potential leguminous winter cover crops include crimson clover, hairy vetch, and lupine. Non-leguminous cover crops include cereals (rye, wheat, barley, oats, triticale), forage grasses (annual ryegrass), and broadleaf species (buckwheat, mustards, and brassicas, including the forage radish).
Additional Tips to Reduce Nitrogen Losses
Consider a fifth R, right irrigation, in addition to 4R nutrient stewardship, as movement of nutrients is governed by soil moisture. Soil moisture sensors for irrigation management will be helpful tools for reducing N leaching.
Account for N contributed by all sources, such as previous crop rotations, irrigation, and rainfall.
Match amounts of N applied to crop needs with split applications timed to support plant growth.
- Consider using precision agriculture tools such as variable rate technology, or NDVI measurements for determining application of fertilizers.
- Avoid applying fertilizers to steeply sloped land or locations near surface water.
- Keep application rates of inorganic fertilizers at lower levels prior to heavy rainfall. Apply split doses of fertilizers to prevent losses in runoff and denitrification.
- Use crop rotations for more efficient use of N. Include legumes to reduce the need for external N inputs and to maintain healthy soils.
Take-Home Points
- Climatic conditions and management practices in row crop production systems in north Florida are highly conducive to N fertilizer losses to the environment through leaching and gaseous emissions (volatilization).
- Following the 4R’s (right source, right rate, right time, and right place) is a best management practice that can help lower N losses and improve crops' N fertilizer recovery.
- Band application of N fertilizers could help lower N fertilizer losses compared to broadcasting.
- EENFs (controlled-release fertilizers or fertilizers with urease and nitrification inhibitors) may reduce NH3 losses from urea, are relatively inexpensive, and can be a potential management practice for protecting against NH3 volatilization.
References
Adotey, N., A. McClure, and X. Yin. 2021. “Enhanced Efficiency Nitrogen Fertilizer As a Tool to Control Nitrogen Loss in Row Crop Production.” PB 1888. https://extension.tennessee.edu/publications/Documents/PB1888.pdf
Cantarella, H., R. Otto, J. R. Soares, and A. G. de Brito Silva. 2018. “Agronomic Efficiency of NBPT As a Urease Inhibitor: A Review.” Journal of Advanced Research 13:19–27. https://doi.org/10.1016/j.jare.2018.05.008
Meng, Y., J. J. Wang, Z. Wei, S. K. Dodla, L. M. Fultz, L. A. Gaston, R. Xiao, J. H. Park, and G. Scaglia. 2021. “Nitrification inhibitors reduce nitrogen losses and improve soil health in a subtropical pastureland.” Geoderma 388:114947. https://doi.org/10.1016/j.geoderma.2021.114947
Silva, A. G., C. H. Sequeira, R. A. Sermarini, and R. Otto. 2017. “Urease Inhibitor NBPT on Ammonia Volatilization and Crop Productivity: A Meta‐Analysis.” Agronomy Journal 109(1): 1–13. https://doi.org/10.2134/agronj2016.04.0200