Purpose and Target Audience
Soil microorganisms, including soil bacteria and fungi, play important roles in pasture systems. They contribute to enhanced soil structure, nutrient cycling, and plant resilience. All these are important to promote healthy and sustainable pastures. Furthermore, soil microbes can act as indicators reflecting the nutritional status of the pasture soil. It is important to assess the impact of agricultural practices on these soil microorganisms for holistic farming. This publication targets a diverse audience, including growers, producers, Extension agents, stakeholders, policymakers, and members of the public who are interested in promoting sustainable agriculture.
Bahiagrass and Rhizoma Perennial Peanut Integration
Warm-season perennial grasses are the pillar of the cow-calf industry in Florida (Figure 1). Bahiagrass (Paspalum notatum Flüggé) is a major warm-season perennial grass that occupies approximately 78% of the total pastureland in Florida. It is desirable for the cow-calf industry because it can tolerate low soil fertility, low soil pH, grazing practices, and a wide range of environmental conditions. To increase the quality and quantity of bahiagrass produced, nitrogen (N) fertilizer application is necessary. Rhizoma perennial peanut (RPP) (Arachis glabrata Benth.) is a warm-season perennial legume that converts atmospheric nitrogen (N) into plant-available N through biological nitrogen fixation (BNF). It has greater nutritional quality for cattle than bahiagrass. The nutritive value of RPP, measured by in vitro digestible organic matter and crude protein, is 40% and 65% greater than that of bahiagrass, respectively. In Florida, the inclusion of RPP into warm-season grass pastures (Figure 1) is being encouraged to reduce reliance on nitrogen fertilizers, mitigate environmental risks, and improve pasture quality and quantity and animal performance at a low N cost.

Credit: Adesuwa S. Erhunmwunse
Rhizoma perennial peanut supplies N to pastures through its mutualistic partnership with bacteria, commonly known as rhizobia, in the root nodules. Although not as efficient as RPP, grasses also form close associations with N-fixing bacteria such as Azospirillum. In addition to N-fixing bacteria, plants interact with other soil microbes (bacteria and fungi), such as arbuscular mycorrhizal fungi and plant growth-promoting microbes that can increase plant nutrient supply, recycle nutrients, increase soil organic matter, provide stress tolerance, and offer disease resistance to plants. Plants can also influence the types of microbes present in the soil of an agricultural system through compounds they release from their roots. The impact of plants on soil microbes varies depending on the plant type and cultivar, location, and season. In bahiagrass-RPP pastures, we can take advantage of the positive effect of both forages on soil microbes and their related plant growth-promoting traits and ecosystem services. This publication discusses soil microbial communities in bahiagrass-RPP pastures in Florida and the impact of pasture locations and seasons on these microbes. Gathering a surveillance database of soil microbial communities in our pastures across different locations and seasons offers valuable insights for tailoring future management practices to specific situations. This may involve adjusting forage integration plans, fine-tuning nutrient management strategies, and adopting practices to enhance both diversity and activity of good microbes.
Common Soil Microbial Indicators in Bahiagrass-RPP Pastures
A healthy soil, free from degradation or contamination, is estimated to contain 1 billion bacteria and 1 million fungi per gram. Globally, there are more than 10,000 bacterial species and 60,000 fungal species. Although most of these microbes have not been cultured in the lab, their roles in agricultural soils have been recognized. Identifying common microbial taxa in any system is important to understand their predictive functions and their contributions to the ecosystem function. Common microbial taxa such as Bacillus, Bradyrhizobium, Rhodoplanes, Fusarium, Trichoderma, and Mortierella found in bahiagrass-RPP pastures (Table 1) have the capability to fix atmospheric nitrogen, enhance plant litter decomposition, improve nutrient cycling, and decrease soilborne plant pathogens.
We have found Bacillus and Fusarium to be the most common bacterial and fungal genera in bahiagrass-RPP pastures in Florida (Table 1). Bacillus is a well-known plant-promoting bacterial genus that contributes to diverse ecological functions in soils, such as fixing atmospheric nitrogen, releasing antimicrobial compounds to inhibit soilborne plant pathogens, and secreting phytohormones to stimulate plant growth and protect from environmental stress like drought. Although Fusarium is commonly thought to be pathogenic, it has diverse functions. It contributes in beneficial ways to pasture soils, aiding in decomposition of organic materials and plant disease suppression.
Mixtures of bahiagrass and RPP can increase soil microbial diversity (i.e., the number of different soil microbes present in soil and the distribution of these soil microbes). Soil fungal diversity and community are easily influenced by bahiagrass-RPP mixtures. However, it requires a longer establishment period of RPP into bahiagrass pastures to observe changes in soil bacterial communities. Erhunmwunse et al. (2023a; 2023b) found an increase in the relative abundance of soil fungal genera, such as Fusarium/Gibberella and Humicola (Figure 2) responsible for N cycling and soil organic matter decomposition, within less than 2 years of incorporating RPP into bahiagrass systems. Meanwhile, increase in soil bacterial diversity was only observed in a well-established (i.e., over 9 years) bahiagrass-RPP system (Figure 3).
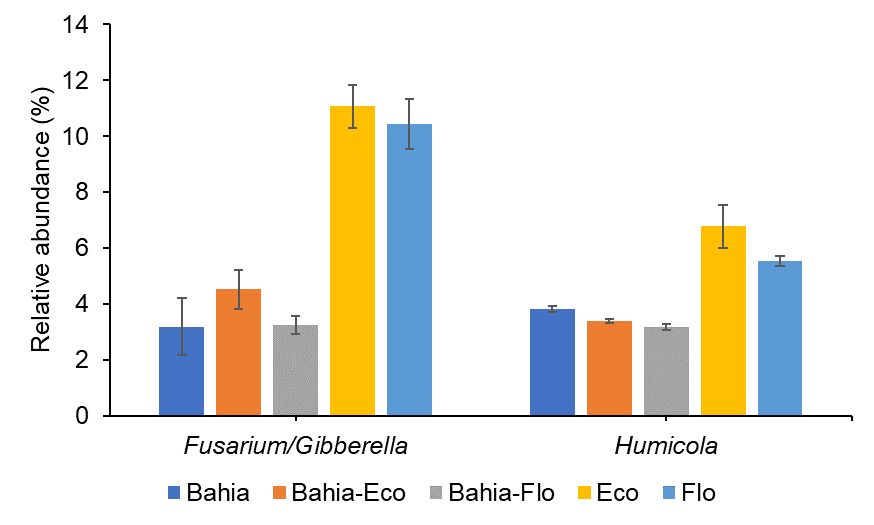
Credit: Erhunmwunse et al. (2023b)
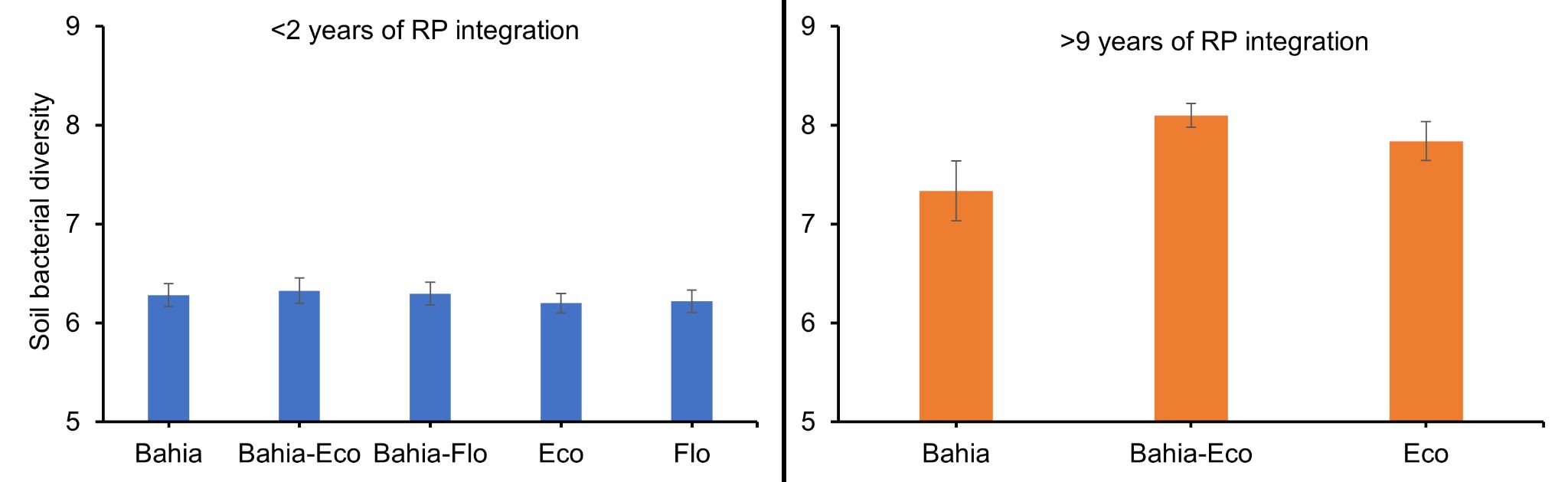
Credit: Erhunmwunse et al. (2023a; 2023b)
Bahiagrass and RPP, when combined in pastures, offer distinct characteristics such as nutritive value and root characteristics, which can affect their impacts on soil microbes. Specific soil microbes responsible for different functions in pasture soils thrive differently under each forage type. For example, RPP enhanced the relative abundance of the bacterial genus Crossiella, which possesses antimicrobial properties that can inhibit soilborne plant pathogens. On the other hand, bahiagrass supported the siderophore-producing bacteria, Arthrobacter. Siderophores are molecules (like tiny magnets) used to take up nutrients such as iron for plants and soil microbes. Mixing bahiagrass and RPP creates a home for diverse beneficial soil microbes needed to build a healthy and sustainable pasture.
Table 1. The dominant microbial indicators in RPP-based bahiagrass pastures and their roles in promoting plant growth and soil health.
Pasture locations impact soil microbial indicators
Forage production differs in quality and quantity across different pasture locations in Florida because of differences in environmental conditions and management practices. This can in turn affect how forages influence soil microbial communities. Three on-farm studies were conducted in north, central, and south Florida to compare the effect of bahiagrass-RPP mixtures vs. bahiagrass system only on soil microbial communities. While the impact of bahiagrass-RPP mixtures on some soil microbes was consistent across pastures in Florida, we found that pasture locations affected some other soil microbes. Bahiagrass-RPP mixtures promoted the relative abundance of the fungal class Nectriaceae (made up of mainly Fusarium) in north and south Florida, but not in central Florida. In north Florida, bahiagrass-RPP soils supported greater relative abundance of arbuscular mycorrhizal fungi than bahiagrass soils. However, there was no impact in central Florida. Additionally, we found a decrease in the relative abundance of arbuscular mycorrhizal fungi in bahiagrass-RPP compared to bahiagrass monocultures in south Florida (Figure 4).
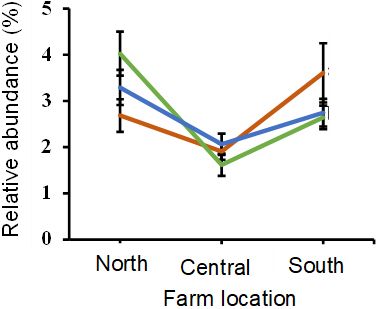
Credit: Erhunmwunse et al. (2023c)
Bahiagrass-RPP mixtures consistently had greater forage production in north and south Florida than in central Florida. These results demonstrate that the impact of RPP on soil microbes will not always be consistent in different pastures. Soil microbes respond to a combination of factors such as forage growth, soil chemical properties (soil pH, phosphorus, and nitrogen), environmental conditions, and animal activities. Soil pH is a major factor contributing to changes in soil microbes in bahiagrass-RPP pasture locations across Florida.
Seasonality affects soil microbial diversity and communities
Seasonal effects on soil microbial communities in pastures are linked to changes in climate conditions and plant growth stages. We observed an increase in soil bacterial diversity in bahiagrass-RPP mixtures only in north Florida in May (Figure 5). There was an increase in soil fungal diversity in bahiagrass-RPP plots across the pastures observed in north, central, and south Florida during peak forage production in July. Generally, fungal diversity responded to RPP integration across all pasture locations in Florida, while soil bacterial diversity was mainly affected by changes in soil properties and climate conditions specific to locations and seasons.
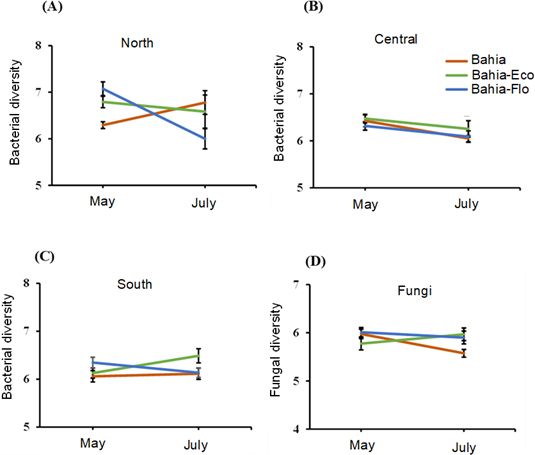
Credit: Erhunmwunse et al. (2023c)
In another study conducted at UF/IFAS NFREC in Marianna, FL, greater fungal diversity in bahiagrass-RPP systems was observed in October compared to March. This increase was associated with greater abundance of fungal phyla, Basidiomycota, Glomeromycota (arbuscular mycorrhizal fungi), and Rozellomycota. Since bahiagrass and RPP are sensitive to daylight duration, forage growth is limited in March compared to October in north Florida. The fungal phyla identified include genera that are responsible for breaking down plant litter. Changes in forage growth during different seasons can influence soil microbial communities.
Summary
- Mixtures of bahiagrass and RPP pastures support greater soil microbial diversity and are associated with unique taxa that are known to promote nutrient cycling and plant litter decomposition.
- Soil fungal communities are easily influenced by bahiagrass-RPP mixtures, while effects on soil bacterial communities are observed when RPP is integrated into bahiagrass systems over time.
- Pasture locations and growing seasons can influence the effect of bahiagrass-RPP mixtures on soil microbial communities.
References
Chambliss, C., and L. Sollenberger. 1991. “Bahiagrass: The Foundation of Cow-Calf Nutrition in Florida.” Proceedings of the 40th Florida Beef Cattle Short Course:74–80.
Erhunmwunse, A. S., V. A. Guerra, J. C. Liu, C. L. Mackowiak, A. R. S. Blount, J. C. B. Dubeux Jr., and H. L. Liao. 2023a. “Soil bacterial diversity responds to long-term establishment of perennial legumes in warm-season grassland at two soil depths.” Microorganisms 11 (12): 3002. https://doi.org/10.3390/microorganisms11123002
Erhunmwunse, A. S., C. L. Mackowiak, A. R. Blount, J. C. B. Dubeux Jr., A. Ogram, and H. L. Liao. 2023b. “Short-Term Perennial Peanut Integration into Bahiagrass System Influence on Soil Microbial-Mediated Nitrogen Cycling Activities and Microbial Co-occurrence Networks.” European Journal of Soil Biology 119:103566. https://doi.org/10.1016/j.ejsobi.2023.103566
Erhunmwunse, A. S., L. M. D. Queiroz, K. Zhang, C. L. Mackowiak, A. R. S. Blount, J. C. B. Dubeux Jr., and H. L. Liao. 2023c. “Changes in Soil Microbial Diversity and Community Composition across Bahiagrass and Rhizoma Peanut Pastures.” Biology and Fertility of Soils:1–16. https://doi.org/10.1007/s00374-023-01701-z
Guerra, V. A., L. Beule, C. L. Mackowiak, J. C. B. Dubeux Jr., A. R. Blount, X. B. Wang, D. L. Rowland, and H. L. Liao. 2022. “Soil Bacterial Community Response to Rhizoma Peanut Incorporation into Florida Pastures.” Journal of Environmental Quality 51 (1): 55–65. https://doi.org/10.1002/jeq2.20307
Jalonen, R., P. Nygren, and J. Sierra. 2009. “Root Exudates of a Legume Tree as a Nitrogen Source for a Tropical Fodder Grass.” Nutrient Cycling in Agroecosystems 85:203–213. https://doi.org/10.1007/s10705-009-9259-6
Prine, G. M., E. C. French, A. R. Blount, M. J. Williams, and K. H. Quesenberry. 2010. “Registration of Arblick and Ecoturf Rhizoma Peanut Germplasms for Ornamental or Forage Use.” Journal Plant Registrations 4:145–148. https://doi.org/10.3198/jpr2009.07.0392crg
Queiroz, L. M. D. 2021. Performance and Nitrogen Dynamics in Grass-Legume Systems in Florida. Dissertation. Gainesville, FL: University of Florida.
Santos, E. R. S., J. C. B. Dubeux Jr., L. E. Sollenberger, A. R. S. Blount, C. Mackowiak, N. DiLorenzo, D. M. Jaramillo, L. Garcia, T. P. Pereira, and M. Ruiz-Moreno. 2018. “Herbage Responses and Biological N2 Fixation of Bahiagrass and Rhizoma Peanut Monocultures Compared with Their Binary Mixtures.” Crop Science 58:2149–2163. https://doi.org/10.2135/cropsci2018.02.0128