Introduction
As technologies for horse selection and breeding evolve, it has become essential for horse owners and breeders to have basic knowledge of equine genetics. Through an understanding of genetic terminology and application of genetics to the equine industry, owners and industry professionals will be equipped to make better decisions based on the information provided by each horse's genetic make-up, or genotype, and to use currently available genetic tools to improve selection.
What is a genome?
Each genome contains all of the instructions needed to perform the functions of life. These instructions are converted into genes and maintained in sets of chromosomes, much like the multiple volumes of an encyclopedia. The genome of the domestic horse is similar in size to that of humans. Like in all living creatures, the genome of the horse is made of DNA, which contains approximately 2.7 billion bases, its building blocks. Of these 2.7 billion bases, approximately 3% contain the instructions for building proteins such as enzymes and other cellular components. We cannot fully decode the remaining 97%, although it likely contains much of the crucial information on when, where, and how each gene product should be used.
The equine genome is organized into 33 chromosomes, 31 of which are autosomes and two of which are sex chromosomes, X and Y, just as in humans (Figure 1). Most cells contain two copies (pairs) of the genome, giving a total of 64 chromosomes (a mirror to the 46 chromosomes in humans). This diploid organization of the genome within the cell allows each individual gene to be present in two full copies (alleles). Thus, if one copy acquires a detrimental mutation (an alternative allele or variant of the gene), the other will be able to continue to make a functional version of the gene product.
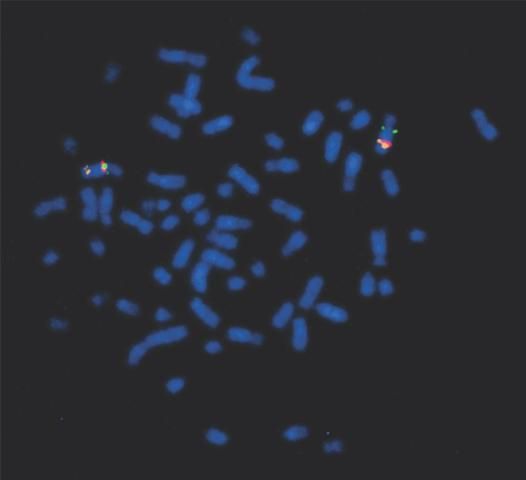
Credit: Samantha A. Brooks, UF/IFAS
The system of duplicate genes also introduces a variety of effects created when the offspring inherits one or two alternative copies from the mother and/or the father. At the most basic level, these interactions are described as recessive or dominant. Should an individual possess two different copies of a gene (a heterozygous genotype), only the effect of the dominant allele will be visible, while the recessive allele will not be expressed. For the effect of a recessive allele to be visible, the horse must possess two recessive alleles. If an individual carries two identical alleles for a gene, recessive or dominant, the genotype is said to be homozygous for that locus.
Armed with knowledge of these patterns of inheritance for a given trait, you can predict the likelihood of obtaining a foal with this trait from a mating of two horses of known genotype. Geneticists use a tool called a Punnett Square to predict the results of a breeding for a single gene trait. Each parent has an equal chance to pass on either of its two alleles for a given gene. For example, if both parents are heterozygous, the distribution of possible genotypes in the resulting offspring will be the same as the result of flipping two coins with three possible combinations of alleles (Figure 2).
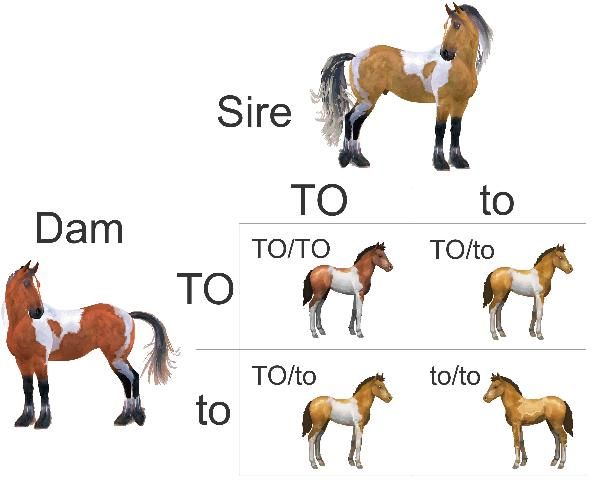
Credit: Laura Patterson Rosa and Samantha A. Brooks, UF/IFAS
How do you determine the genotype of a horse?
For certain traits there are guidelines (and myths) for making an educated guess regarding genotype. However, with the completion of the horse genome sequence in 2009 and accelerated research efforts, we can now test for more alleles in the horse than ever before. A brief list of currently available genetic tests in the horse is presented in Table 1 (reviewed in Bailey, Brooks, and Bowling 2013). Breeders can effectively use these tests to improve their foal crop and therefore increase the profitability of their operation, or decrease the frequency of tragic and costly genetic conditions. Nonetheless, the use of these tests is not yet widespread in the industry. Many of these tests are recent discoveries, and only a few companies provide an efficient system to get this information out to the industry. The cost of testing can also be a significant deterrent. On average, testing costs $20–$50 per trait in each horse. However, strategic testing can minimize the number of horses that need to be examined. In addition, emerging technologies will begin to rapidly decrease the cost per test.
What should I breed for?
Choosing a stallion for your mare is the most important business decision of your breeding program. The first step should always be a careful evaluation of the qualities and faults of the mare, including any relevant genetic testing. If you know in advance that your mare carries an allele for a recessive condition, then you can avoid the production of a sick foal by utilizing a stallion free of that allele. Identifying a list of desired traits in your foal crop will aid in the selection of relevant tests and stallions.
While the popularity of a stallion can increase the value of his foals, choice of a stallion based solely on the marketability of his name is risky. Overuse of popular stallions and the associated increase of linebreeding or inbreeding can have a detrimental effect on the long-term health of the breed. For example, the American Quarter Horse is the world's largest breed. It is well-known for the range of activities in which it excels. However, within specific subgroups of the breed, a popular sire effect (when a single individual is bred repeatedly, reducing genetic diversity in the population) has led to increases in the frequency of genetic conditions. An example is the mutation called HYPP, the first mutation discovered in the horse. In 1992, when a genetic test for this allele became available (Rudolph et al. 1992), the frequency of the disease in halter-bred Quarter Horses was 22%. However, despite the availability of the genetic test, which permits the selection of breeding stock that is free of the disease, the frequency of HYPP increased to 56.4% in the halter-bred Quarter Horse in just 17 years (Tryon et al. 2009). The genetic test was used primarily to reduce the clinical signs by avoiding production of homozygous foals. Consequentially, the number of heterozygous carriers has increased dramatically. This strategy capitalizes on a short-term gain for the horse operation, but leads to a long-term loss for the quality of the breed as a whole. It is also important to remember that the popularity of a stallion may rise and fall, but the value of a sound, healthy, and quality horse is fundamentally stable. In the end, a trendy pedigree may mean nothing for a foal affected with a genetic condition.
What is next for genetic tools?
To date, research has focused on single-gene conditions and colors. With improved tools facilitated by the horse genome sequence, we can begin to evaluate more complex traits like performance, conformation, and behavior. These same tools will also change the way we approach genetic testing. Most companies offer testing on only one allele at a time or a small bundle of two to three alleles. Novel methods allow testing of many genes in one test, and are used by some genetic testing companies for a fraction of the cost.
Genetic testing is not just for the horse breeder. Tests for conditions like HYPP and PSSM1 can be utilized as a diagnostic tool, providing you and your veterinarian with answers that may help to make key management and treatment decisions to improve the health and well-being of your horse. Genetic testing is also a great educational tool, for example, in determining the precise color or pattern of your horse. Products to determine the breed of a mixed-breed dog are now commonplace, and are both fun and educational. A comparable service is not yet available for the horse, but it may be available soon.
Unlike other animal diagnostic tests, the evaluation of a horse's genome is often instigated by the horse owner instead of the veterinarian. It is important for the horse owner to keep this in mind and to carefully consider integrating genetic testing into their management and breeding programs. Hopefully, the field of equine genomics will continue to be a front-runner in the creation of new and valuable tools for the horse industry.
Summary
The use of genetic tools to improve selection of horses is rapidly becoming a reality for horse owners, breeders, and veterinarians. Many traits of interest can now be selected for, and genetic diseases can be diagnosed or eliminated from the breeding program utilizing genotype-assisted selection. For additional information on equine genetics and ongoing research, visit the Brooks Equine Genetics Laboratory website at http://www.ufequinegenetics.org.
References
Bailey, E., S. A. Brooks, and A. T. Bowling. 2013. Horse Genetics. Wallingford, Oxfordshire, UK; Boston, MA: CABI.
Rudolph, J. A., S. J. Spier, G. Byrns, C. V. Rojas, D. Bernoco, and E. P. Hoffman. 1992. "Periodic paralysis in Quarter Horses: a sodium channel mutation disseminated by selective breeding." Nature Genetics 2: 144–7.
Tryon, R. C., M. C. Penedo, M. E. McCue, S. J. Valberg, J. R. Mickelson, T. R. Famula, M. L. Wagner, et al. 2009. "Evaluation of allele frequencies of inherited disease genes in subgroups of American Quarter Horses." J. Am. Vet. Med. Assoc. 234: 120–5.Table 1.
Commercially available genetic tests for the horse (Bailey, Brooks, and Bowling 2013).