Introduction
This Ask IFAS publication is directed to beef producers, farmworkers, and Extension agents with the purpose of providing information regarding current pregnancy diagnosis methods for beef cattle.
The most recent survey by the USDA explains that only 31.6% of beef producers in the United States use any pregnancy diagnosis tool (NAHMS 2020). Identifying productive beef females within the herd allows producers to manage their bottom line. In the beef industry, a standard expectation is that females should produce one calf every year. By using pregnancy diagnosis, producers can identify the pregnancy status of females within ±30 days after breeding instead of waiting for the entire gestation (±283 days). This allows producers to choose whether they rebreed or cull the animal (Youngquist 2007).
Is it necessary to make this decision? Is it worth spending money on the diagnosis? Suppose the pregnancy status is unknown and the cow is maintained in the herd instead of culled. The total annual economic losses of keeping infertile cows could be between $2,800 and $13,200 per 100 head cow herds, depending on the operation (Prevatt et al. 2018). This is a significant amount of money for any producer, and such losses could have a negative effect on profitability.
For producers doing artificial insemination (AI) protocols, an early pregnancy diagnosis could be an opportunity for a second round of AI in the subsequent estrous cycle. Additionally, early rebreeding means a higher profit due to earlier calving at the beginning of the calving season and calves that are older and heavier at the time of weaning (i.e., more pounds to sell). Lastly, cows will have more time to recover from calving before the next breeding season (Gonella 2020).
Rectal Palpation
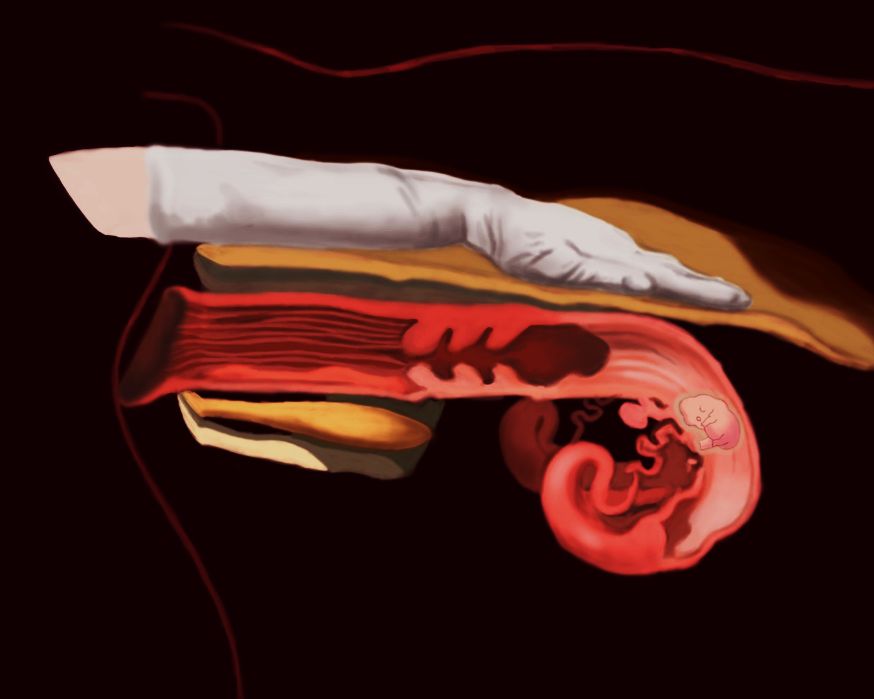
Rectal palpation is the oldest, simplest, and most commonly used method in beef cattle. It requires a trained technician or a veterinarian to ensure accuracy, but results are available in real time.
The diagnosis can be made anytime from 30 to 40 days after breeding, depending on the technician’s skill, until the pregnancy is full term (±283 days). The technician will use their skills to find the uterus and horns of the cow through its rectum (Figure 1). After localizing the structures, the technician will identify changes in the anatomy, such as size of the uterine horns, fluid in the uterine horns, fetal membranes, and placentomes (union between the placenta and the uterus). Depending on the age of the pregnancy, even the fetus may be identified (Taverne and Noakes 2018; Youngquist 2007). In animals with fewer than 45 days post-breeding, it is recommended that rectal palpation only be performed by licensed veterinarians or embryologists due to the risk of causing abortion.
As with any test, there is no 100% accuracy for the result given by the technician. Certain conditions could be palpated and mistaken for pregnancy, such as incomplete uterus involution, pyometra (infection in the uterus), mucometra (mucus in the uterus), and hydrometra (water in the uterus). Additionally, an inexperienced technician could mistake other structures such as the rumen or a kidney for the uterus (Taverne and Noakes 2018; Youngquist 2007). The cost of rectal palpation for pregnancy diagnosis is highly variable. Some producers learn how to do it, and others call technicians or licensed veterinarians to do it. We have estimated that the cost could vary from $2 to $10 per head (Griffith 2018; Marshall et al. 2022).
Ultrasound
Over the last decade, ultrasonography has become more widely used in the cattle industry and recommended for pregnancy diagnosis (Filho et al. 2020; Peixoto et al. 2021). As with transrectal palpation, the results are given at the time of the exam. Technician skills and training increase the accuracy of the diagnosis using ultrasound (Balhara et al. 2013).
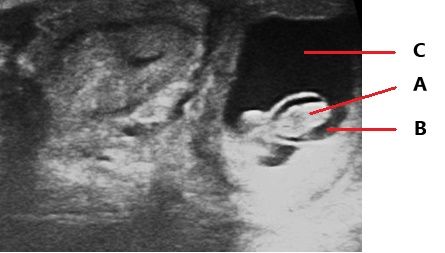
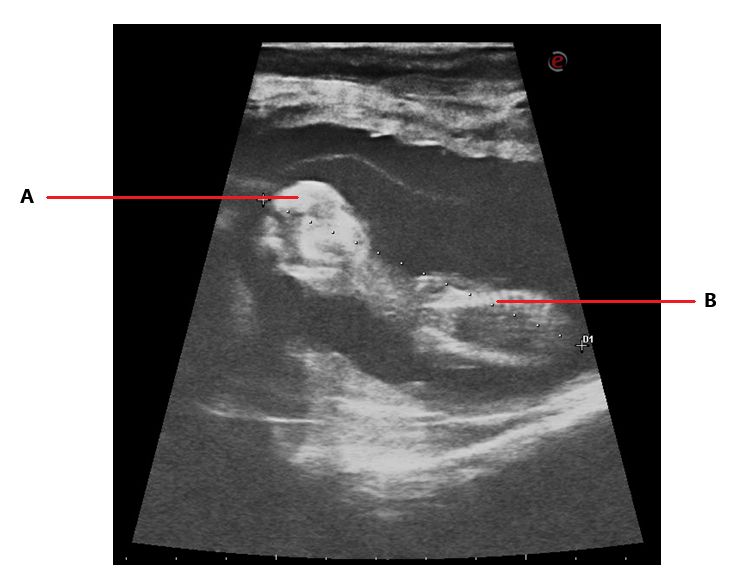
This exam can be performed with accurate results starting at 28 days of gestation. The procedure is like rectal palpation with the difference that the technician will introduce the ultrasound probe with their hand in the rectum of the cow and will look at the monitor of the ultrasound to find the image of the uterus and the horns. After that, with soft movements the technician will look for signs of pregnancy. The presence of fluid (Figure 2), a small fetus (Figure 2) or a completely differentiated fetus (Figure 3), the heartbeat of the embryo, and embryonic membranes can indicate pregnancy (Szenci 2021; Youngquist 2007).
Another advantage of this exam is that it can provide more information such as the age of the pregnancy, size of the fetus, sex, twins, status of the ovaries, and more (Balhara et al. 2013; Fricke et al. 2016; Taverne and Noakes 2018).
An incorrect diagnosis is still possible, depending on the skills of the technician, the time of the pregnancy during which the exam is performed, and conditions that result in an accumulation of fluid in the horns (Taverne and Noakes 2018). This method is about as accurate as rectal palpation, but it can be used for an earlier diagnosis. The cost of ultrasonography for pregnancy diagnosis is highly variable. Some producers purchase their own ultrasound units and learn how to do it. In other operations, they call a licensed veterinarian to do it. It has been estimated that pregnancy diagnosis using ultrasound could cost between $10 and $20 per head, but this cost could vary depending on the price of the equipment (Griffith 2018; Marshall et al. 2022).
Blood Test
Some specific proteins can be detected in cows’ blood during pregnancy. The most commonly used protein for the identification of pregnant females is pregnancy-associated glycoproteins (PAGs), which are released by the placenta and can be detected by day 26 after conception (Filho et al. 2020).
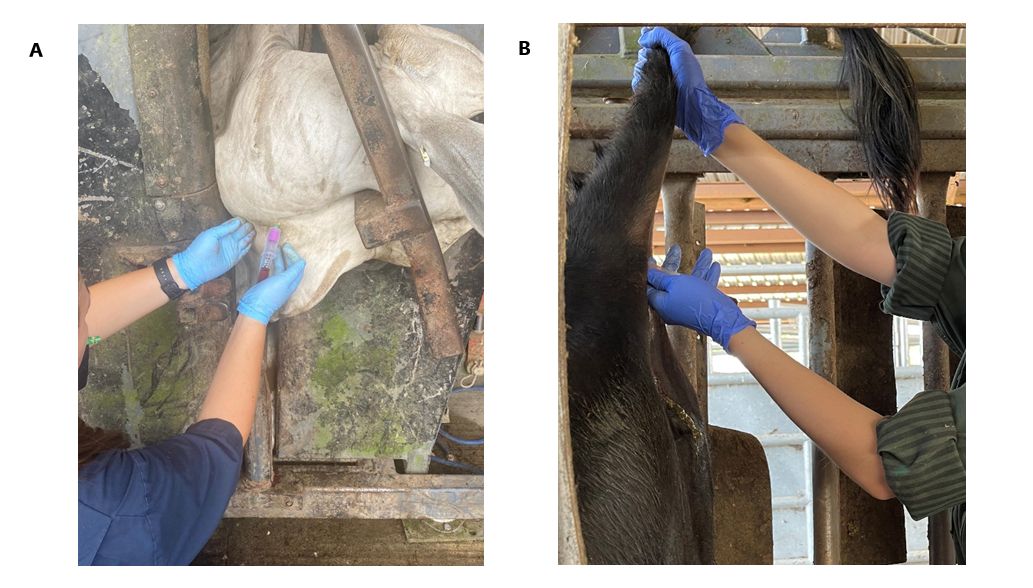
There are several commercial tests available for producers to purchase. To perform any of these tests, you need a small sample of blood (2 to 5 cc or mL) from the jugular vein in the neck (Figure 4A) or from the coccygeal vein in the tail (Figure 4B). Samples should be taken in an EDTA tube and labeled with the cow ID. Depending on the test type, blood samples will be sent to a laboratory (Figure 5) or processed chute-side (Figure 6). Depending on the test, the results can be ready in 20 minutes to a couple of days. The average cost of the test per animal is approximately $5; this cost could vary depending on the number of samples and the chosen test (Szenci 2021).
The results of this test are very accurate (95%–99%), but the precision will depend on the age of the pregnancy, parity of the cow, days post-partum, presence of twins, embryonic mortality, and milk production (Pohler et al. 2016).
Conclusions
Every cow-calf operation can benefit economically by incorporating one of the described pregnancy diagnosis strategies into herd management; if a producer has 100 cows and, after the breeding season, does not do a pregnancy diagnosis, and 20% of cows are open until the calving season, the producer would be spending around $6,300 to feed the open cows. However, if the producer uses the blood test and takes the open cows out of the herd, the producer will spend $500 on the test and save $5,800 just in feed. Therefore, it is important for producers to use pregnancy diagnoses on their herds to identify open cows and make the most convenient management decisions. Infertile cows are a waste of resources, and it is vital for the beef operation to identify those animals and remove them from the herd (Prevatt et al. 2018).
Credit: UF/IFAS NFREC Repro Lab (2022)
Credit: UF/IFAS NFREC Repro Lab (2022)
Credit: UF/IFAS NFREC Repro Lab (2022)
Credit: UF/IFAS NFREC Repro Lab (2022)
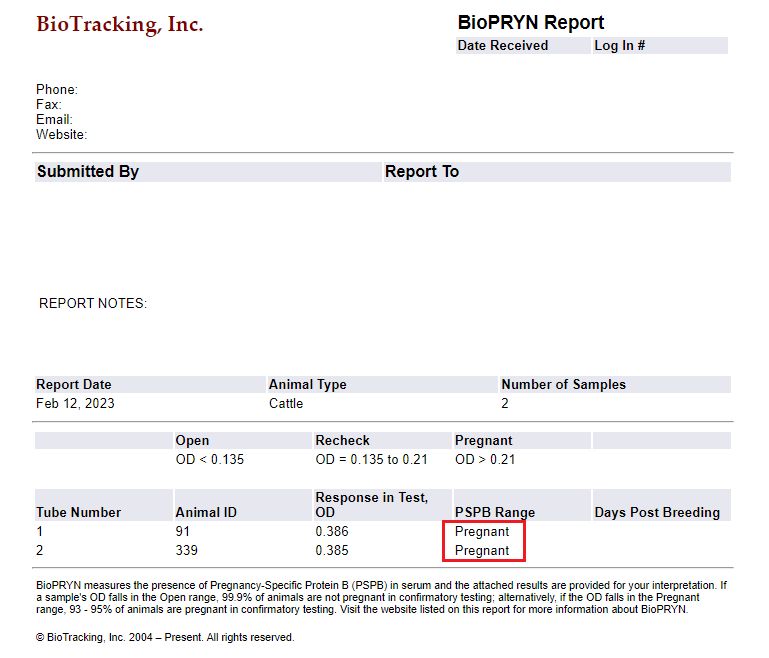
Credit: UF/IFAS NFREC Repro Lab. Results of a real blood test from BioTracking© (2023)
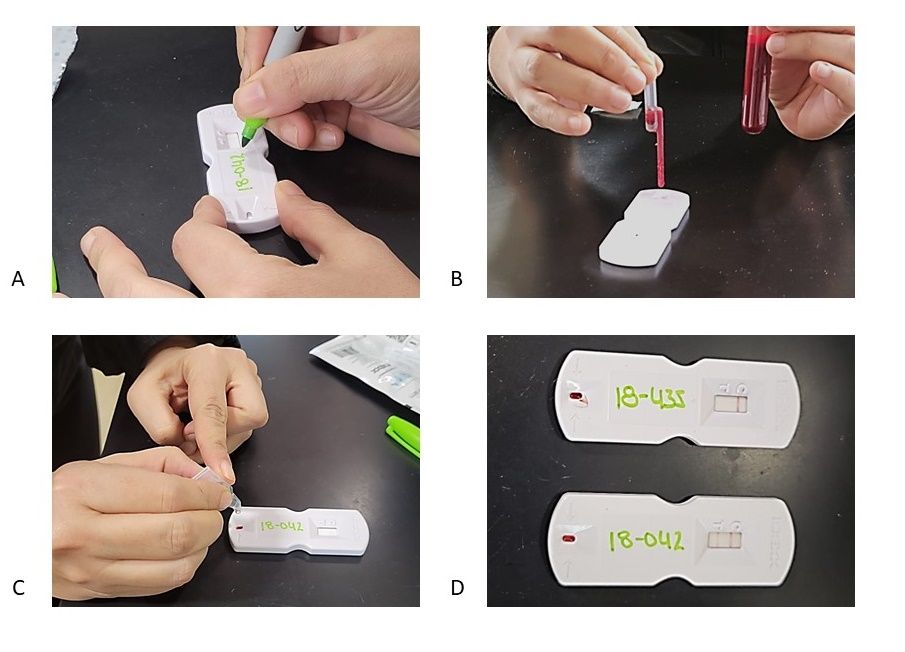
Credit: UF/IFAS NFREC Repro Lab. Idexx (2023)
References
Balhara, A. K., M. Gupta, S. Singh, A. K. Mohanty, and I. Singh. 2013. “Early Pregnancy Diagnosis in Bovines: Current Status and Future Directions.” The Scientific World Journal 2013. https://doi.org/10.1155/2013/958540
Filho, R. V. O., G. A. Franco, S. T. Reese, F. G. Dantas, P. L. P. Fontes, R. F. Cooke, J. D. Rhinehart, K. W. Thompson, and K. G. Pohler. 2020. “Using Pregnancy Associated Glycoproteins (PAG) for Pregnancy Detection at Day 24 of Gestation in Beef Cattle.” Theriogenology 141:128–133. https://doi.org/10.1016/j.theriogenology.2019.09.014
Fricke, P. M., A. Ricci, J. O. Giordano, and P. D. Carvalho. 2016. “Methods for and Implementation of Pregnancy Diagnosis in Dairy Cows.” Veterinary Clinics of North America - Food Animal Practice 32 (1): 165–180. https://doi.org/10.1016/j.cvfa.2015.09.006
Gonella, A. 2020. “Honey, we need to talk about calving distribution.” The Florida Cattleman and Livestock Journal:62–63.
Griffith, A. 2018. “The cost of keeping one open cow can pay to have the herd pregnancy checked.” Ohio BEEF Cattle Letter. https://u.osu.edu/beef/2018/06/27/the-cost-of-keeping-one-open-cow-can-pay-to-have-the-herd-pregnancy-checked/
Marshall, T., J. Maples, and K. Coatney. 2022. “Benefit-Cost Analysis of Selling Pregnant Replacement Females: Information and Timing Matters.” Mississippi State University Extension. https://extension.msstate.edu/publications/benefit%E2%80%93cost-analysis-selling-pregnant-replacement-females-information-and-timing
Peixoto, P. M., A. M. Hubner, W. M. C. Junior, L. L. Cunha, E. F. Garrett, K. G. Pohler, N. W. Dias, V. R. G. Mercadante, I. F. Canisso, and F. S. Lima. 2021. “Characterization of Pregnancy-Associated Glycoproteins and Progesterone as a Predictor of Twins and Conceptus Loss in High-Risk-Pregnancy Holstein Cows.” Journal of Dairy Science 104 (4): 5034–5046. https://doi.org/10.3168/jds.2020-19334
Pohler, K. G., R. F. G. Peres, J. A. Green, H. Graff, T. Martins, J. L. M. Vasconcelos, and M. F. Smith. 2016. “Use of Bovine Pregnancy-Associated Glycoproteins to Predict Late Embryonic Mortality in Postpartum Nelore Beef Cows.” Theriogenology 85 (9): 1652–1659. https://doi.org/10.1016/j.theriogenology.2016.01.026
Prevatt, C., G. C. Lamb, C. Dahlen, V. R. G. Mercadante, and K. Waters. 2018. “What is the economic impact of infertility in beef cattle?: AN208, rev. 9/2018.” EDIS 2018 (4). https://doi.org/10.32473/edis-an208-2018
Ricci, A., P. D. Carvalho, M. C. Amundson, R. H. Fourdraine, L. Vincenti, and P. M. Fricke. 2015. “Factors Associated with Pregnancy-Associated Glycoprotein (PAG) Levels in Plasma and Milk of Holstein Cows during Early Pregnancy and Their Effect on the Accuracy of Pregnancy Diagnosis.” Journal of Dairy Science 98 (4): 2502–2514. https://doi.org/10.3168/jds.2014-8974
Sasser, R. G. 1986. “Detection of Pregnancy by Radioimmunoassay of a Novel Pregnancy- Specific Protein in Serum of Cows and a Profile of Serum Concentrations during Gestation.” Biology of Reproduction 35 (4): 936–942. https://doi.org/10.1095/biolreprod35.4.936
Szenci, O. 2021. “Recent Possibilities for the Diagnosis of Early Pregnancy and Embryonic Mortality in Dairy Cows.” Animals 11 (6). MDPI AG. https://doi.org/10.3390/ani11061666
Taverne, M., and D. E. Noakes. 2018. “Pregnancy and Its Diagnosis.” In Veterinary Reproduction & Obstetrics. 78–114. Elsevier. https://doi.org/10.1016/B978-0-7020-7233-8.00005-7
Youngquist, R. S. 2007. “Pregnancy Diagnosis.” In Current Therapy in Large Animal Theriogenology. 294–303. https://doi.org/10.1016/B978-072169323-1.50042-8
Zoli, A. P., L. A. Guilbault, P. Delahaut, W. B. Ortiz, and J.-F. Beckers. 1992. “Radioimmunoassay of a Bovine Pregnancy-Associated Glycoprotein in Serum: Its Application for Pregnancy Diagnosis.” Biology of Reproduction 46 (1): 83–92. https://doi.org/10.1095/biolreprod46.1.83