Introduction
Praxelis clematidea is a newly emerging weed species in Florida, one that Plant Protection and Quarantine (PPQ) is considering adding to the federal noxious weed list (USDA-APHIS 2014). It was first discovered in an abandoned orange grove in Orange County, Florida, in 2006 (Abbott et al. 2008). The plant can be easily misidentified and confused with Ageratum houstonianum (bluemink) and Conoclinium coelestinum (blue mistflower) as well as several other species that have similar flower characteristics. This article is written for green industry professionals and others to aid in the identification and management of praxelis in and around ornamental plants.
Species Description
Class
Dicot
Family
Asteraceae
Other Common Names
Praxelis
Life Span
Annual or short-lived perennial.
Habitat
Praxelis grows in tropical and subtropical environments and is native to South America (Vedkamp 1999). It tolerates partial to full sun but does not grow well in fully shaded conditions (CRC Weed Management 2003). It is usually found growing in disturbed areas of roadsides, in pastures, along railway lines, in recently burned areas, in open woods, and along fence lines or streambanks (Waterhouse 2003). In the nursery, it grows in noncrop areas (walkways, aisles, dry ditch banks), on container media surfaces (in container-grown ornamentals), and in pot drain holes.

Credit: Annette Chandler, UF/IFAS
Distribution
Praxelis is native to Argentina, Bolivia, southern Brazil, and some other parts of South America (King and Robinson 1970). It has been introduced and become naturalized in China and Taiwan, and it is especially problematic in Australia, where it has begun invading natural areas as well as agricultural production fields (USDA-APHIS 2014). Praxelis has been documented in seven central Florida counties (Hardee, Hillsborough, Lake, Manatee, Orange, Osceola, and Polk) (Wunderlin and Hansen 2014). This species is thought to have been introduced into Florida as a contaminant in commercial trade of seeds or possibly landscaping products, such as mulch and building materials (CRC Weed Management 2003). The predicted or potential distribution of praxelis includes most of the southeastern United States as well as Hawaii (USDA-APHIS 2014).
Growth Habit
Praxelis is an upright-growing herb that commonly grows to a height of 16–20 inches but has been observed growing as tall as 40 inches (Abbott et al. 2008).
Seedling
Seedlings are light green in color. The cotyledon (seed leaves) are hairy, are toothed along the leaf margins (serrated), and have noticeable midveins.

Credit: Annette Chandler, UF/IFAS
Shoots
The stem is cylindrical, brittle, green in color, and covered in hair (pubescence). The leaves are hairy underneath, oppositely arranged, and approximately 1 to 2.5 inches long and 0.5 to 1.5 inches wide. The shape of leaves ranges from a tear to a diamond, and they have large-toothed edges with 5–8 large teeth (or serrations) on each side. One of the distinguishing features of praxelis is the smell of its leaves, which emit a foul odor like cat urine when crushed or after mowing (CRC Weed Management 2003).

Credit: Annette Chandler, UF/IFAS

Credit: Annette Chandler, UF/IFAS
Roots
Praxelis has a fibrous root system.
Inflorescence
Praxelis has lavender- or bluish-colored flowers that are in clusters of about 35–40 tubular florets (tiny flowers) growing in groups at the ends of hairy stems. The key distinctive feature of praxelis is that florets are set into a highly cone-shaped receptacle. Flowering mostly occurs from January to May, but plants have been observed flowering throughout the year in central Florida when winter temperatures remain mild (above freezing).

Credit: Annette Chandler, UF/IFAS
Fruits and Seeds
The seeds are black in color and 0.1–0.2 inches long. Seeds have a "pappus" or a cluster of barbed bristles (15–40 bristles) that can help them spread by wind or water, or by attaching themselves to animal fur and feathers, clothing, or machinery.

Credit: Annette Chandler, UF/IFAS
Similar Species
Praxelis clematidea is morphologically similar to several other species in Florida, such as the native species Chromolaena odorata (Siamweed) and Fleischmannia incarnata (pink thoroughwort), and two other related exotic weed species, Ageratum conyzoides (tropical whiteweed) and Ageratum houstonianum (bluemink) (CRC Weed Management 2003, Gardner and Williges 2015). This similarity and misidentification between praxelis and ageratum led to the unreported spread of praxelis in Australia and Hong Kong (Gardner and Williges 2015) for several years. It is probably the same reason praxelis remained unreported in Florida until recently (Abbott et al. 2008). The leaves in Ageratum houstonianum are broader and heart-shaped (cordate), and their finely or bluntly edged teeth on the leaf blade (Figure 7) are smaller than those of praxelis, which has more diamond-shaped leaves with larger teeth or serrations (Figure 7). The bract that surrounds and supports the flower head is persistent, unlike the corresponding bract in praxelis. The plant also lacks pappus, and seeds consist of four to five small, bristle-like scales. Ageratum littorale (Cape Sable whiteweed) and Conoclinium coelestinum (blue mistflower) are two other native species that look similar to Praxelis clematidea. Ageratum littorale and Conoclinium coelestinum tend to grow in sandy soils along the coast and prefer moist to wet soils.
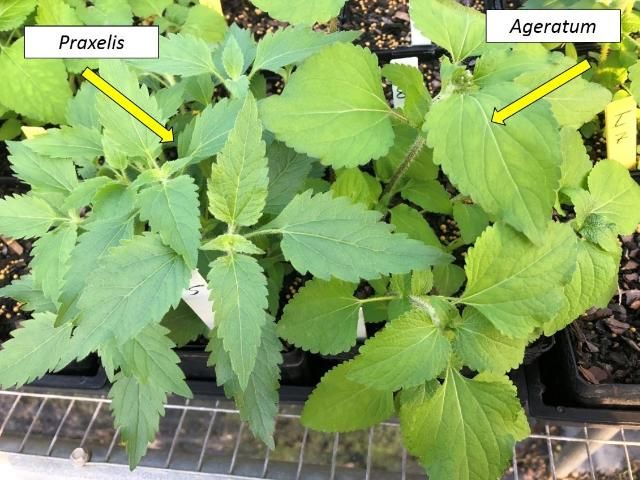
Credit: Chris Marble, UF/IFAS
Management
Physical and Cultural Control
In containers, praxelis should be hand weeded before it starts flowering. Each plant can produce thousands of seeds in a few months after germination. Scouting and regular hand weeding will prevent any further spread of the plant. In addition to hand weeding, the use of mulch such as pine bark, wood chips, rice hulls, or weed disks has proven to be effective on many weeds. In noncrop areas, such as walkways and aisles between container production spaces, mowing can reduce seed production and spread.
Chemical Control
Preemergence Herbicides
Due to its relatively new emergence and the fact that it is currently only found in Florida, praxelis is not listed as a controlled weed on any preemergence herbicide labeled for use in nurseries. Research at the University of Florida has shown that preemergence herbicides, including oxyfluorfen + prodiamine (Biathlon), flumioxazin (Broadstar or SureGuard), and oxyfluorfen + pendimethalin (OH2), provided excellent control of praxelis. A similarity among all of these products is that each contains a protoporphyrinogen oxidase (PPO) inhibitor (Group 14) herbicide as at least one of the active ingredients. Other herbicides that provided control or suppression were indaziflam (Marengo® SC) and isoxaben + prodiamine (Gemini® SC). Products that provided poor control include dimethenamid-P (Tower® and as a component of FreeHand®) and isoxaben + trifluralin (Snapshot®). Preemergence herbicides that have been evaluated for control of praxelis are listed in Table 1 along with efficacy rankings for the rates that were evaluated.
Postemergence Herbicides
The State of Queensland Department of Agriculture, Fisheries, and Forestry (DAFF) has recommended the use of herbicides such as glyphosate (RoundUp® Pro and many other trade names), fluroxypyr (Vista®), metsulfuron-methyl (Escort® XP), 2,4-D (many trade names), and picloram (Tordon®) as potential options for praxelis control, but the effectiveness of these herbicides on praxelis has not been reported in the literature (DAFF 2014). It is also important to note that most of these active ingredients would only be labeled for use in noncrop areas and would not be options near ornamental plants. Research at the University of Florida has shown that herbicides including triclopyr (Garlon® 4), clopyralid (Lontrel®), and glyphosate (Ranger® PRO) can provide 80% to 100% control for 12 weeks. Glufosinate (Finale®) also provided control for 4 to 6 weeks, but regrowth and recovery was much more rapid, likely due to the limited translocation of glufosinate compared with the other herbicides that were evaluated. Due to the high seed production of praxelis, more long-term control would likely be achieved by combining the use of preemergence and postemergence herbicides to prevent regrowth from germination.
References
Abbott, J., C. L. White, and S. Davis. 2008. "Praxelis clematidea (Asteraceae), a Genus and Species New for the Flora of North America." Journal of the Botanical Research Institute of Texas 2:621–626.
Animal and Plant Health Inspection Service (APHIS). 2014. "Risk Assessment for Praxelis clematidea." https://www.aphis.usda.gov/plant_health/plant_pest_info/weeds/downloads/wra/Praxelis_clematidea.pdf. Accessed Nov. 12, 2019.
CRC Weed Management. 2003. "Weed Management Guide: Praxelis (Praxelis clematidea)." Cooperative Research Centre (CRC) for Australian Weed Management, Australia.
Department of Agriculture, Fisheries, and Forestry (DAFF). 2014. "Praxelis (Praxelis clematidea)." PP113. The State of Queensland, Australia. http://www.daff.qld.gov.au/__data/assets/pdf_file/0019/63172/IPA-Praxelis-PP113.pdf. Accessed Oct. 24, 2019.
Gardner, A. G., and K. A. Williges. 2015. "Praxelis clematidea (Asteraceae): A New Plant Invader of Florida." Southeastern Naturalist 14 (1).
King, R. M., and H. Robinson. 1970. "Studies in the Eupatorieae (Compositae). XXVIII. The Genus Praxelis." Phytologia 20:193–195.
Kumar, S., and C. M. Singh. 1989. "Control of Ageratum conyzoides L. under Mid Hill Conditions of Himachal Pradesh." Indian Journal of Weed Science 21:55‒59.
Singh, C. M., N. M. Angras, and S. Kumar. 1996. Weed Management. New Delhi, India: MD Publications.
Veldkamp, J. F. 1999. "Eupatorium catarium, a New Name for Eupatorium clematideum Griseb., non Sch.Bip. (Compositae), a South American Species Naturalised and Spreading in SE Asia and Queensland, Australia." Garden Bulletin Singapore 51:119–124.
Waterhouse, B. M. 2003. "Know Your Enemy: Recent Records of Potentially Serious Weeds in Northern Australia, Papua New Guinea and Papua (Indonesia)." Telopea 10:477–485.
Wunderlin, R. P., and P. F. Hansen. 2014. Atlas of Florida Vascular Plants. University of South Florida, Department of Biology, Institute for Systematic Botany. http://florida.plantatlas.usf.edu/Default.aspx. Accessed June 7, 2019.
Table 1. Preemergence herbicides labeled for use in ornamental plant production and evaluated for control of praxelis in Florida research trials*.
Table 2. Postemergence herbicides and their efficacy for control of mature and flowering praxelis in experiments conducted in Florida*.