Target Audience
This publication is intended to provide information to professional landscapers, pest control operators, Extension agents, and homeowners on how to recognize and potentially manage anthracnose diseases of landscape plants.
Introduction
Fungi make up approximately 90% of known plant pathogens. One of the most common fungal diseases of plants is anthracnose. The name anthracnose represents a group of related fungal diseases that affect many different landscape and crop plants. These anthracnose diseases produce similar sets of symptoms with a few consistent characteristics. Though members of several genera are known to cause anthracnose, the most causative agents belong to the Colletotrichum genus. In Florida, C. gloeosporioides is the most common species causing anthracnose in the landscape. The disease can be a significant problem during stressful, hot, and humid conditions.
Life Cycle
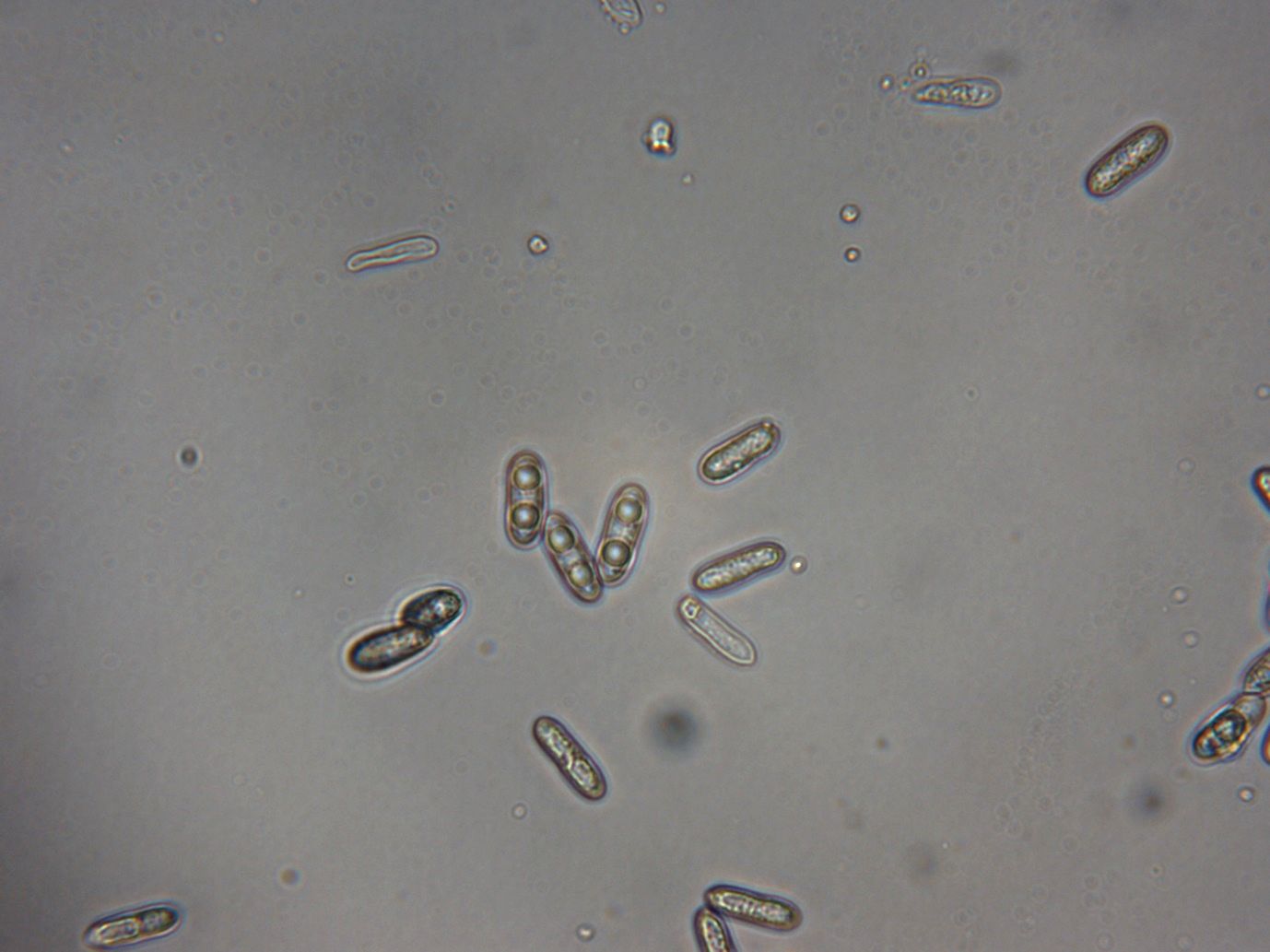
The Colletotrichum species that cause anthracnose are common in the Florida landscape. When not causing disease, they help decompose dead or damaged plant tissues, acting as saprophytes (living on dead plants). These fungi produce spores that are sticky and not easily spread through the air. Instead, water splashing from rainfall and irrigation more commonly transports the spores to their host. They may spread from diseased or decaying plant tissue to healthy plants from people’s hands or tools. If the fungal spores (Figure 1) settle on the surface of a host plant, the right level of moisture allows them to produce short hyphae, which terminates in anchoring and penetrating structures called appressoria. The appressoria produce penetration pegs that work their way through the cuticle of living plants into the epidermis and deeper plant tissues. For a time, the invading hyphae may feed on living cells without damaging them (biotrophy). The young hyphae may also go into quiescence, a dormant period in the fungal life cycle that can extend from days to months. Therefore, in a biotrophic or quiescent stage, the pathogen does not produce any noticeable symptoms. Its presence may be confirmed with molecular lab analyses.
Physical damage, chemical burns, or stress from notable environmental changes in temperature or moisture can initiate the active phase of the disease cycle. Extended periods of wet and humid weather in the form of rain, dew, or fog produce environmental conditions that favor fungal growth and increase the plant’s susceptibility to disease. When leaf surfaces remain wet for four or more hours after an irrigation event or rainstorm, the anchored fungus begins to kill plant tissues and feed from the resulting dead (necrotic) plant organs. Even moisture from dew can extend the time the plant remains wet, increasing its chances to infiltrate plant tissues. Although a wound on an infected plant is not necessary for the pathogen to enter its host, physical damage can increase how severe the disease symptoms may become. As lesions form on the diseased plant parts, the pathogen continues to produce more of the sticky spores that continue the cycle of infection if favorable conditions persist.
Symptoms and Signs
Anthracnose can cause symptoms on a diverse range of plant parts, including leaves, stems, fruits, and flowers. Anthracnose causes death and discoloration of plant tissues and may be confused with numerous other plant diseases or damages. Salt burn from excess salinity can produce anthracnose-like symptoms, such as tip and marginal leaf burn, leaf browning, and defoliation. Sunscald may be mistaken for anthracnose as well. It is important to keep in mind that, in many cases, symptoms can have multiple causes simultaneously (abiotic damage and multiple diseases on the same plant parts).
Anthracnose symptoms vary depending on the fungal species complex, the host plants, and weather conditions. The following list describes a range of typical symptoms of the disease, usually occurring in various combinations:
- Brown-to-black spots appear on leaves surrounded by yellow halos and concentric rings.
- Irregular yellow or brown spots on leaves darken and merge as they age, sometimes covering the leaves.
- Leaves and tender shoots are blighted by lesions and withering.
- Chlorotic-to-necrotic leaf burn starts with a bright yellow color on the margins. As the chlorosis progresses toward the center and lower end of the leaf, the marginal leaf tissue turns brown and becomes distorted.
- Marginal leaf burn and leaf spots occur simultaneously.
- Large areas of dead tissue cause cupping and distortion of expanded leaves.
- Twigs and branches die back.
- Leaf loss occurs in severe cases and seasonally. Leaves regenerate with new growth.
- Small, dark, sunken lesions of irregular shape appear on fruit and spread as the disease progresses, degrading the quality of maturing fruit, which eventually rots on the tree or in storage.
Anthracnose rarely causes severe and lasting damage to plants. For some plants, the infection is a seasonal occurrence and part of the replacement of old leaves with new ones. This commonly occurs with queen crape myrtle (Lagerstroemia speciosa, Figure 45) and many of the trumpet trees (Handroanthus spp.; Tabebuia spp., Figure 44). Other plants, most noticeably the corn plant (Draceana fragrans, Figure 20), seem unable to rid themselves of the disease because of persistent and unfavorable environmental conditions.
Susceptibility and severity of anthracnose infections may increase when plants, poorly adapted to wet conditions, are growing in moisture-retaining areas in the landscape. However, anthracnose-causing fungal species may be part of the microflora of landscape plants. Changes in environmental conditions, the onset of leaf senescence, nutrient deficiencies, or a decline in the plant’s overall health may facilitate the fungi’s transition from commensal or mutualistic symbiont (not harmful or even beneficial to the plant host) to opportunistic pathogen (causing disease because of the decline in plant health).
Signs of the anthracnose pathogen are more difficult to see than the symptoms on the plant. These include structures and spores produced by the pathogen on symptomatic plant tissues. The most distinct anthracnose signs are hair-like structures called setae (singular: seta) (Figure 2). Visible with a magnifying glass, setae are distinctly black in color and arranged in small circular groups of protruding spore-producing structures called acervuli. Setae are not always present. They may be cultivated and observed by enclosing symptomatic plant material in a resealable bag with a moist paper towel for 24 hours (incubation).
Masses of sticky spores may also be visible after incubation or extended periods of wet conditions. Colletotrichum spores frequently form orange-to-salmon-colored clusters and can form tendrils that exude from blister-like structures embedded in the host plant’s dead tissue (Figure 3).
Management
These diseases are often effectively controlled by following good sanitary and cultural practices and are rarely serious enough to warrant chemical control. The following are ways to manage the disease:
Manual
- Increase air circulation by removing some plants or spacing them further apart.
- For herbaceous perennial and palms, remove and discard diseased leaves depending on species tolerance.
- For woody perennials and trees, prune out infected stems and branches about four inches below diseased areas, using a sanitizer on pruning tools between cuts.
- Rake and remove fallen leaves.
- Increase sun exposure or decrease shade.
- Decrease volume or duration of irrigation depending on species recommendations.
- Restrict irrigation or watering to morning hours after dewfall to reduce the duration the plant is wet.
- Install drip irrigation (or micro-irrigation) or carry out manual watering to prevent wetting leaves, stems, and fruits.
- Keep records of diseased species and times of occurrence. Symptoms of anthracnose often are seasonal, appearing with the natural cycle of senescence and disappearing with the flush of new growth.
Chemical
- Several fungicides are available that provide varying levels of control of anthracnose on ornamental plants. Fungicides can only protect healthy tissue and do not cure existing infections. During periods of wet, humid weather typical of the rainy season that favor spore dispersal and active infections, consider applying fungicides according to the shorter interval listed on the product label (usually 7 to 10 days). Spread fungicide applications over longer intervals when drier conditions are unfavorable for fungal infection. Plants showing resistance to or tolerance of anthracnose infection may not require fungicide applications or may only need a seasonal preventative treatment.
- Protective fungicides recommended for managing Colletotrichum spp. of ornamental plants contain chlorothalonil (Daconil Ultrex, etc.), Mancozeb (Manzate Max, etc.), or copper sprays containing copper diammonia diacetate or copper octanoate. The systemic fungicides thiophanate-methyl (Cleary’s 3336, for professional use only), chlorothalonil + thiophanate methyl (Spectro 90WDG), pyraclostrobin + boscalid (Pageant, etc.), and tebuconazole (Tebuzol 3.6F, intended for professional applicators) offer the greatest control if applied before disease development. For more information on other fungicide products and active ingredients, see EDIS publication PP154, “Homeowner’s Guide to Fungicides for Lawn and Landscape Disease Management.” Read and follow all label directions.
- Systemic fungicides are not recommended or labeled for edible plants in the landscape since the residual toxic compounds, or their metabolites, may persist in the edible fruit and vegetative structures. Some product labels specify that treated fruit should not be consumed (ornamental only). Read and follow all label directions carefully.
Table 1. A partial list of landscape plants affected by Colletotrichum spp.
Credit: Philip F. Harmon, UF/IFAS

Credit: Philip F. Harmon, UF/IFAS

Credit: Philip F. Harmon, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Ryan Czaplewski, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Romina Gazis-Seregina, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Ian Maguire (emeritus), UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS

Credit: Stephen H. Brown, UF/IFAS
References
Crane, J. H., C. F. Balerdi, and I. Maguire. 2006. “Mango Growing in the Florida Home Landscape: HS2/MG216, rev. 10/2005.” EDIS 2006 (18). https://doi.org/10.32473/edis-mg216-2006
DeSilva, D. D., P. W. Crous, P. K. Ades, K. D. Hyde, P. W. J. Taylor. 2017. “Lifestyles of Colletotrichum Species and Implications for Plant Biosecurity.” Fungal Biology Reviews 31 (3): 155–168. https://doi.org/10.1016/j.fbr.2017.05.001
Douglas, S. M. 2011. Anthracnose Diseases of Trees. The Connecticut Agricultural Experiment Station. https://portal.ct.gov/-/media/caes/documents/publications/fact_sheets/plant_pathology_and_ecology/anthracnosediseasesoftrees060311rpdf.pdf
Downer, A. J., S. S. Swain, and A. C. Crump. 2020. “Home and Landscape: Anthracnose.” UC IPM. UC ANR Publication 7420. https://ipm.ucanr.edu/home-and-landscape/anthracnose/pest-notes/#gsc.tab=0
Growables, Inc. 2013. “Avocado Diseases.” Growables: Grow Florida Edibles. Last revised May 2, 2020. https://www.growables.org/information/TropicalFruit/AvocadoDiseasesInsects.htm
Nelson, S. 2008. “Anthracnose of Avocado.” Publication No. PD-58. University of Hawaii at Manoa, Cooperative Extension Service. https://www.ctahr.hawaii.edu/oc/freepubs/pdf/pd-58.pdf
Nelson, S. C. 2008. “Mango Anthracnose (Colletotrichum gloeosporioides).” Publication No. PD-48. University of Hawaii at Manoa, Cooperative Extension Service. https://www.ctahr.hawaii.edu/oc/freepubs/pdf/pd-48.pdf
Pernezny, K., and R. B. Marlatt. 2007. “Diseases of Avocado in Florida.” PP21. University of Florida Institute of Food and Agricultural Sciences. https://ufdcimages.uflib.ufl.edu/IR/00/00/28/56/00001/VH04700.pdf
Popenoe, J., C. R. Warick, and D. J. Norman. 2021. “Key Plant, Key Pests: Lilyturf (Liriope muscari): EP600/ENH1336, 3/2021.” EDIS 2021 (2). https://edis.ifas.ufl.edu/publication/EP600
Weir, B. S., P. R. Johnston, U. Damm. 2012. “The Colletrotrichum gloeosporioides Species Complex.” Studies in Mycology 73 (1): 115–180. https://doi.org/10.3114/sim0011