Introduction
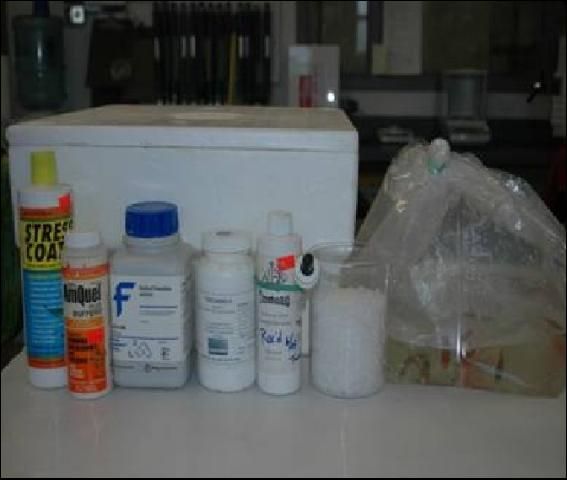
The purpose of this article is to instruct ornamental aquaculture producers how to safely transport ornamental fish within their aquaculture facility. Any trauma and stress associated with handling and transport of fish will affect survival and overall quality of the fish (see UF/IFAS Circular 919 Stress-It's Role in Fish Disease). Fish should be moved quickly and efficiently to minimize stress, the risk of disease outbreaks, and mortality. Important considerations for transporting fish include: 1) type of container, 2) transport vehicle, 3) aeration, 4) type of water, and 5) additives for sedating the fish (Figure 1).
Credit: Tina Crosby (2004)
The purpose of this article is to instruct ornamental aquaculture producers how to safely transport ornamental fish within their aquaculture facility. Any trauma and stress associated with handling and transport of fish will affect survival and overall quality of the fish (see UF/IFAS Circular 919 Stress-It's Role in Fish Disease). Fish should be moved quickly and efficiently to minimize stress, the risk of disease outbreaks, and mortality. Important considerations for transporting fish include: 1) type of container, 2) transport vehicle, 3) aeration, 4) type of water, and 5) additives for sedating the fish (Figure 1).

Credit: Tina Crosby (2004)
Transport Considerations
Many factors including dissolved oxygen levels, changes in temperature, and pH differences between transport and holding water should be considered when transporting fish. Poor water conditions can adversely affect the immune system of the fish, increase susceptibility to disease, and may lead to illness or death. Determine these differences before moving the fish, and, if necessary, make adjustments to the water. Then acclimate the fish. Acclimation is the process of slowly introducing the fish to different quality water to allow physiological adjustments to occur gradually over time.
Water Selection
One of the most important factors in moving fish from a pond to the holding facility is the source of water used in the transport container. Water taken directly from the pond, aerated well water, a half-pond / half-aerated well water mixture, or treated municipal water can be used to transport the harvested fish.
Many water quality parameters should be considered when handling fish, including pH, ammonia, nitrite, dissolved oxygen, temperature, total alkalinity, total hardness, and free carbon dioxide. Table 1 shows general desired water quality parameters for freshwater fish. However, different species of fish may have very different water quality requirements, so it is important to know the requirements for each species being transported to ensure its good husbandry and health.
Well water is usually low in dissolved oxygen and may have high concentrations of dissolved carbon dioxide (CO2), dissolved hydrogen sulfide (H2S), or dissolved iron. Vigorous aeration of well water in a mixing vat prior to use helps degas (drive off) dissolved carbon dioxide and hydrogen sulfide and increases the oxygen levels. Before using well water, it is best to test the water (pH, TAN, NO2, DO, alkalinity, hardness, CO2) to ensure good water quality.
Municipal water is generally treated with chlorine or chloramines for disinfection. Because these chemicals are highly toxic to fish, they must be removed from the water before use. Chlorine can be removed using sodium thiosulfate (7.4 ppm sodium thiosulfate for each ppm of chlorine) or with vigorous aeration (Boyd 1990). Chloramine is a combination of chlorine and ammonia. Sodium thiosulfate and aeration does NOT remove chloramines. A commercial product must be used to break down chloramines.
Experiments conducted at the University of Florida Tropical Aquaculture Laboratory demonstrated that fish transported from a pond to a holding facility in aerated well water or a mixture of half pond and half aerated well water had improved behavior (i.e., showed less signs of stress) over time compared to transfer in pond water alone. Well water alone may be warmer or cooler than pond water depending on the season, and it usually lacks dissolved oxygen unless aerated. An advantage of using well water is that it is essentially pathogen-free, and with a bit of management, it can be properly adjusted for use in transporting fish. By contrast, pond water may contain phytoplankton, insects, crayfish, and many potential disease-causing organisms. The mixture of pond water and aerated well water allows the farmer to reduce problems associated with each.
Dissolved Oxygen Levels During Transport: The Limiting Factor
Temperature
Fish are ectothermic, meaning they have a variable body temperature that fluctuates with the temperature of the surrounding environment. Different fish species have different optimal temperature ranges in which they will grow and flourish. Temperature affects respiration, feeding, and digestion. Sudden temperature changes stress the fish, suppress its immune system and can lead to disease and death.
Sunlight can quickly lead to increased transport water temperature, which affects other water chemistry (e.g., ammonia and dissolved oxygen). Additionally, high temperatures can directly cause stress to coolwater fish such as koi and goldfish. In cold weather, the temperature of transport water can drop significantly causing stress to warmwater fish (e.g., swordtails, gouramis, tetras). Thus, it is important to know the temperature tolerances of the species being grown and to compensate as needed.
During transport, temperature differences between transport water and holding facility water can vary greatly. Sudden changes in temperature can result in temperature shock syndrome in fish (Wedemeyer 1996). For this reason, fish must be acclimated (adjusted) to the water temperature in the holding facility.
Acclimating Fish into Holding Systems
The process of acclimation involves placing the transport container in front of or in the holding tank, adding an airline, and gradually adding water from the holding tank into the transport container. The airline will help maintain an adequate dissolved oxygen levels and mixing the waters will prevent rapid temperature and pH changes. If the temperature difference is greater than 10°C, then ideally, acclimation should proceed slowly over a period of two or more hours (Wedemeyer 1996). However, more rapid acclimation may be tolerated by some species.
Effects of Carbon Dioxide and pH on Ammonia Toxicity
High levels of carbon dioxide lowers the affinity of oxygen to bind with hemoglobin in the blood of the fish (Wedemeyer 1996). In water, carbon dioxide forms carbonic acid (H2CO3). As the levels of carbon dioxide and carbonic acid increase, the pH of water decreases. Due to increases in CO2, pH often drops while fish are in transit. Levels of pH under 7 are considered acidic and over 7 are basic. Changes in pH shift in the ratio of unionized to ionized ammonia.
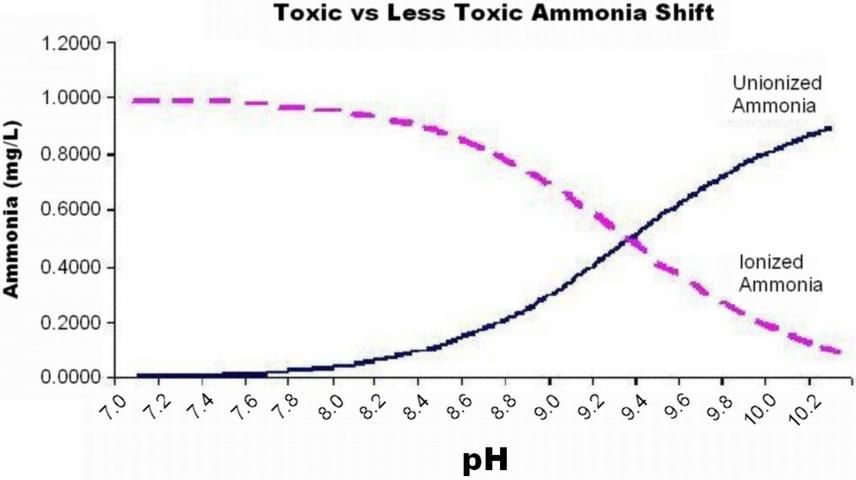
Ammonia is a metabolic waste product released primarily thorough the gills of fish. At high concentrations, ammonia can cause gill damage and stress (see UF/IFAS Fact Sheet FA-16 Ammonia). As transport time or the number of fish in the transport container increase, the amount of total ammonia nitrogen (TAN) accumulates in the water. Total ammonia nitrogen is present in two forms: ionized ammonia (IA) and unionized ammonia (UIA). Unionized ammonia (NH3) is much more toxic than ionized ammonia (NH4+). The portion of total ammonia that is present as toxic unionized ammonia (UIA) shifts with changes in temperature and pH of the water. As temperature and pH increase, the ammonia shifts to the more toxic form.
In transport water, carbonic acid produced by release of carbon dioxide lowers the pH of the water and shifts the ratio of toxic unionized ammonia to the less toxic ionized form. Figure 3 shows the shift between the unionized (toxic) and ionized (less toxic) forms of ammonia with change in pH, for a water sample at 75°F, with 1.0mg/L TAN.
Credit: Tina Crosby (2004)
Chemical Additives
Sedatives and salt are widely used in transport water to aid in alleviating stress and trauma to fish. Stress causes a rise in blood cortisol levels. Cortisol is a steroid hormone that causes physiological (e.g., increased heart rate, increased ventilation) and metabolic (i.e., energy) changes. The fish diverts energy to restore its physiological balance, which can lead to reduced immunity against disease. Sedatives slow down the metabolism of the fish which reduces respiration and ultimately helps the fish compensate for some fluctuations in water quality (e.g., low dissolved oxygen). Decreased respiration and activity lessens oxygen demand as well as the possibility of fish abrading themselves against the transport container and each other.
Salt reduces physiological stress by decreasing the osmotic gradient between the fish and the transport water, thus decreasing the amount of energy the fish must use for osmoregulation.
Additives can improve the quality of the shipped fish. Some farmers choose to treat fish with a prophylactic antimicrobial chemical during transport, while others wait until the fish have been acclimated to conditions in the holding facility (see UF/FAS Fact Sheet FA-120 Preparation and Packaging of Ornamental Fish for Shipping). Before using any chemical as a prophylactic treatment, a history of problems should be established. Using a chemical, especially an antibiotic, without a real need is wasteful, can result in increased bacterial resistance to antibiotics used, and can be detrimental to the fish.
On-farm transport trials were run at the University of Florida Tropical Aquaculture Laboratory to evaluate some commonly used additives. Fish were trapped in a pond and put into a polystyrene transport container with half pond water, half aerated well water. The boxes of fish were then transported by truck for four hours. Treatments tested were oxygenation with an oxygen bottle, salt (sodium chloride), acriflavine neutral, methylene blue, clove oil, tricaine methanesulfonate (MS-222), quinaldine, and no treatment (control) (Table 2).
Upon arrival back at the facility, the fish were put into tanks with flow-through aerated well water, and held one week for observation. Using MS-222 improved the appearance (e.g., less ragged finnage, more normal coloration, minimal scale-loss) of the fish immediately following transportation from the pond; however, after one week of being in the holding facility, the appearance of the fish was no better than those that received no treatment. The behavior of the fish treated with MS-222 was no different than other treatments tested. All other treatments, except acriflavine neutral, had no beneficial or negative effect. The use of acriflavine neutral was found to have a negative effect on fish. Fish transported with acriflavine neutral looked worse (e.g., clamped fins, scale-loss, torn fins, etc.) than other fish and experienced greater mortalities than fish transported with no additive (i.e., control fish).
Depending upon length of time for transport, there may not be a significant benefit to the use of a sedative or salt. Some of these treatments, however, may be useful for extended transportation and shipping of ornamental fish (see UF/IFAS Fact Sheet FA-120 Preparation and Packaging of Ornamental Fish for Shipping).
Summary
Reducing the factors that contribute to stress in ornamental fish during transport from the grow-out pond to the holding facility lowers mortality and improves appearance. Optimal conditions can be achieved with careful planning and preparation prior to the move. Important considerations include 1) transport container, 2) transport vehicle if needed, 3) type of water to be used in the transport container, 4) aeration, and 5) additives. Close attention should be given to various water quality parameters and their interactions. Transport time and fluctuations in water quality should be kept to a minimum. The level of dissolved oxygen and temperature are important and should always be a concern to the farmer. Fish should be slowly acclimated if there are significant differences in transport water and receiving water temperature [i.e., > 5.5°C (10°F)] (Petty et al. 2004). With experience and care, farmers can increase the quality and survival of fish going to market.
Recommended Reading
UF/IFAS Circular 919 Stress - Its Role in Fish Disease. http://edis.ifas.ufl.edu/FA005
UF/IFAS Fact Sheet FA-7 Fish Fingerlings: Purchasing, Transporting, and Stocking. http://edis.ifas.ufl.edu/FA013
UF/IFAS Fact Sheet FA-16 Ammonia in Aquatic Systems. http://edis.ifas.ufl.edu/FA031
UF/IFAS Fact Sheet FA-117 Harvesting Ornamental Fish From Ponds. http://edis.ifas.ufl.edu/FA117
UF/IFAS Fact Sheet FA-118 Grading Ornamental Fish.http://edis.ifas.ufl.edu/FA118
UF/IFAS Fact Sheet FA-120 Preparation of Ornamental Fish for Shipping. http://edis.ifas.ufl.edu/FA120
SRAC Publication No. 410 Calculating Treatments for Ponds and Tanks. https://srac.tamu.edu/categories/view/20
SRAC Publication No. 462 Nitrite in Fish Ponds https://srac.tamu.edu/categories/view/25
SRAC Publication No. 463 Ammonia in Fish Ponds. https://srac.tamu.edu/categories/view/25
SRAC Publication No. 464 Interactions of pH, Carbon Dioxide, Alkalinity, and Hardness in Fish Ponds. https://srac.tamu.edu/categories/view/25
SRAC Publication No. 468 Carbon Dioxide in Fish Ponds. https://srac.tamu.edu/categories/view/25
SRAC Publication No. 474 The Role of Stress in Fish Disease. https://srac.tamu.edu/categories/view/26
SRAC Publication No. 3900 Anesthetics in Aquaculture. https://srac.tamu.edu/categories/view/18
SRAC Publication No. 4601 Measuring Dissolved Oxygen Concentration in Aquaculture. https://srac.tamu.edu/categories/view/25
References and Further Reading
Boyd, C.E. (1990). Water Quality in Ponds for Aquaculture. Birmingham Publishing Company, Birmingham, Alabama.
Petty, B.D., and R.F. Floyd. (2004). Pet fish care and husbandry. Veterinary Clinics Exotic Animal Practice 7:397–419.
Wedemeyer, G.A. (1996). Interactions with Water Quality Conditions. In Physiology of Fish in Intensive Culture Systems. Chapman and Hall, New York, New York.