General Description
The pigfish, Orthopristis chrysoptera (Figure 1), is a member of the grunt family, Haemulidae. As the name implies, members of this family make a "grunting" or "chattering" noise when agitated by rubbing their pharyngeal teeth together. Pigfish are listed as one of the top candidate species for marine baitfish aquaculture by Oesterling et al. (2004). The ovate-elliptical body is considerably compressed with body depth being 30–38% of standard length (Lindeman and Toxey 2003). Coloration of the species can vary from a light blue-grey to light brown dorsally, gradually fading to silver below and occasionally possessing irregular vertical bars or a dark brown mottled appearance, particularly on the head (Darcy 1983; Sutter and McIlwain 1987; Lindeman and Toxey 2003). Rarely, members of a population will display narrow, horizontal dark bars stretching the length of the body. Each scale of the pigfish has a blue center with a bronze spot on the edge (Sutter and McIlwain 1987; Lindeman and Toxey 2003). These spots form stripes, which above the lateral line trail slightly upwards and below the lateral line extend horizontally (Sutter and McIlwain 1987; Lindeman and Toxey 2003). Fins are yellow-brown with dusky, dark margins (Darcy 1983; Sutter and McIlwain 1987; Lindeman and Toxey 2003).
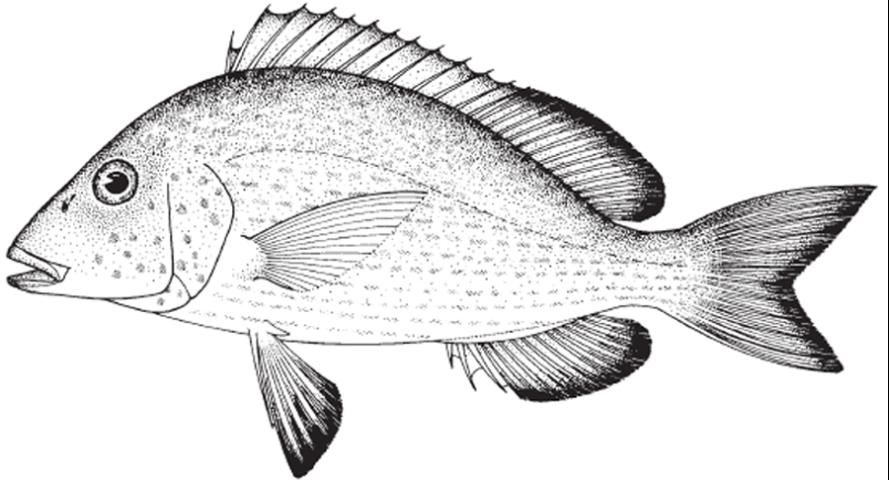
Credit: Food and Agriculture Organization of the United Nations
Geographic Distribution and Habitat
Pigfish occur in the Gulf of Mexico from Florida to the Yucatan peninsula and on the Atlantic coast of the United States from New York to the northern Bahamas and Bermuda, but are less common north of Virginia (Darcy 1983; Sutter and McIlwain 1987; Lindeman and Toxey 2003; Oesterling et al. 2004). Juvenile pigfish typically inhabit shallow, near-shore waters, and are often associated with seagrass beds. Adults occur more frequently on deeper flats over soft bottom habitats, such as channel edges and sandy, sparsely vegetated areas; they can also be found on midshelf reefs (Darcy 1983; Sutter and McIlwain 1987; Lindeman and Toxey 2003).
Natural History
Pigfish can tolerate water temperatures between 13.7 (56.7°F) to 36.0°C (96.8°F), but favor a more moderate temperature of 26.0°C (78.8°F), often migrating offshore when nearshore temperatures fluctuate beyond this range (Darcy 1983; Sutter and McIlwain 1987). They also tolerate salinities from 0 to 44.1 g/L, but will avoid waters less than 15 g/L (Darcy 1983; Sutter and McIlwain 1987). Adults have a maximum recorded total length (TL) of 380 mm (14.9 inches), but rarely exceed 230 mm (9 inches). They also have a recorded lifespan of 4 years, reaching sexual maturity by the end of the second year (Darcy 1983; Sutter and McIlwain 1987; Oesterling et al. 2004). The smallest mature specimens recorded had a TL of 200–215 mm (Darcy 1983). Spawning occurs from late winter to early spring in protected coastal waters or in offshore waters prior to inshore migration (Darcy 1983; Sutter and McIlwain 1987). Pigfish consume a variety of prey throughout their lifespans. Larvae and young juveniles (under 30 mm TL) are planktivorous, feeding primarily on copepods, shrimp larvae, and mysid shrimp (Carr and Adams 1973; Adams 1976; Howe 2001). A gradual shift to a carnivorous diet begins at 30 mm TL; at this stage, pigfish will consume various benthic animals such as polychaetes, amphipods, fish larvae, and eventually shrimp and larger crabs (Carr and Adams 1973; Adams 1976; Howe 2001). Pigfish are prey for larger fish such as snappers, groupers, sharks, and spotted seatrout (Darcy 1983; Sutter and McIlwain 1987; Oesterling et al. 2004). For this reason, they are often caught with traps, seines, and handlines for use as live bait (Sutter and McIlwain 1987).
Culture Techniques
Limited information is available on the aquaculture methods and systems used to rear pigfish. However, studies have been conducted on life history and habitat, feeding, spawning, and larval development, and these studies should help us to develop culture methods for the production of pigfish.
Broodstock
Pigfish reach sexual maturity by the end of the second year, and although wild-caught adults rarely exceed 4 years of age, the longevity of the species in captivity is unknown (Darcy 1983; Sutter and McIlwain 1987; Oesterling et al. 2004). Spawning can occur from January to June, depending on location, with the greatest frequency in March and April (Darcy 1983; Sutter and McIlwain 1987). Within this single spawning season, however, females are thought to spawn multiple times (i.e., they release their eggs in batches) (Darcy 1983). Spawning takes place at dusk in offshore areas prior to inshore migrations or in calm, near-shore waters such as harbors and inlets, where larvae are often found (Darcy 1983; Collins and Finucane 1984; Sutter and McIlwain 1987; Warlen and Burke 1990). Generally, the larger fish will spawn earlier in the season, followed by the smaller fish spawning later in the season (Darcy 1983).
Within the culture system, temperature, salinity, and light cycle may be manipulated to simulate natural seasonal changes and to condition broodstock to spawn. Pigfish are a candidate for strip-spawning, where male and female gametes are gently expressed from the fish and mixed together in seawater, initiating fertilization (Figure 2). Hormones may also be used to induce spawning in broodstock. Pigfish have been successfully induced to spawn in experimental trials using Ovaprim® (Western Chemical, Inc.), an injectable sGnRHa solution, at UF/IFAS Indian River Research and Education Center (IRREC). However, this product is only allowed for use in ornamental and aquarium fish. Chorulon® (Intervet Schering-Plough Animal Health, Inc.), an injectable product containing human chorionic gonadotropin (HCG), is labeled for finfish, but trials have not been conducted to determine if it will cause ovulation in pigfish. Prior to injection, fish were anesthetized with a 50 mg/L dose of MS-222 (tricaine methanesulfonate) to reduce the chance of injury. Ovaprim doses of 0.50 mL/kg female body weight and 0.25 mL/kg male body weight were administered following manufacturer instructions. Spawning at 26°C (78.8°F) and 35 g/L salinity occurred within 48 hours of injection. The eggs are buoyant in seawater and were collected by sieving water skimmed from the surface through a mesh-screened collection device concentrating the eggs (unpublished data). The corocoro grunt, Orthopristis ruber, a cousin to the pigfish, has also been induced to spawn using LHRHa at a dose of 50 ng/kg female body weight and 25 ng/kg male body weight (Mata et al. 2004). Spawning began 28 hours after injection with an average fecundity of 120,000 eggs/female (Mata et al. 2004).

Credit: Eric J. Cassiano, UF/IFAS
Development of an optimal broodstock diet is essential to successful larval rearing, as nutrients are passed from adult to eggs. Wild adult pigfish are opportunistic carnivores consuming a wide variety of benthic invertebrates including shrimp, crabs, and bivalves (Darcy 1983; Sutter and McIlwain 1987). As with most culture fish, broodstock nutrition affects egg and larval quality. Brood diets have not been evaluated with pigfish, so it is recommended that a combination of pelleted 45% protein pelleted feed be provided with fresh or frozen shrimp, squid, and an oily fish such as menhaden being a supplement for months prior to spawning. An examination of the nutritional content of wild pigfish larvae could divulge the proper nutrients needed for a broodstock diet and subsequent optimal larval development. Pigfish broodstock at IRREC were fed a combination of a 2.0 mm slow-sinking, pelleted commercial diet (containing 50% crude protein and 15% crude lipid) and frozen squid.
Hatchery
Once fertilized, the eggs are incubated under similar water conditions to the spawning tank (26.0°C [78.8°F] and 35.0 g/L) in static 200–300 L cylindrical tanks with gentle aeration. Hatching begins within 30 hours of spawning, and larvae will initially subsist on yolk sac protein and lipid reserves provided from the broodstock (Figure 3). By the end of day two post hatch (PH), the yolk sac is depleted and feeding must begin (Watson 1983). Many marine fish larvae are too small to feed on Artemia nauplii and are not able to utilize an artificial feed; therefore, a feeding regime of copepods and/or rotifers must be implemented during the hatchery phase (Stottrup and McEvoy 2003). Also on day two PH, microalgae is added to the culture system to darken the water, regardless of the live feed utilized. Microalgae levels should be maintained between 200,000–1,000,000 cells/mL (18–28 cm secchi depth), depending on the algae species used. This will ensure proper feeding background for fish larvae as well as help meet the nutritional requirements of the live feed and fish larvae (Stottrup and McEvoy 2003). At the IRREC, live Tahitian strain Isochrysis galbana (T-ISO) was maintained between 200,000–500,000 cells/mL (~28 cm secchi depth) during larval feeding. Daily samples of the larval tank will ensure that proper algal and live feed densities are maintained as the pigfish larvae grow. Once feeding begins, water exchange becomes necessary with the implementation of a recirculating or flow-through system.

Credit: Eric J. Cassiano, UF/IFAS
Copepod nauplii are an optimal food source for first-feeding pigfish larvae; they provide the proper nutrition, are of adequate size, and are the primary food source for wild larval pigfish (Carr and Adams 1973; Adams 1976). Replicated research studies have not been performed, but observations suggest advantages in growth, survival, and stress resistance through the use of copepod nauplii fed to pigfish larvae. In Mata et al. (2004), corocoro grunt larvae were fed a mixture of rotifers and copepods, Apocyclops distans, during the larval phase and survived to day 17 PH, the duration of the trial. Rotifers have been used for years as a primary and supplemental live feed for the larval stage of many marine fish species. Although numerous feeding protocols exist, no published data are available on feeding protocols for pigfish larvae (Stottrup and McEvoy 2003). The use of rotifer enrichments is recommended to ensure that the fish larvae will receive the proper nutritional components needed for successful development. Once enriched, rotifers should be stored at approximately 9°C. Cool temperatures decrease their metabolic rate and hence slow the passage of the enrichment product through their gut tracts; otherwise the enrichment passes through too quickly and will contribute to the deterioration of the water quality.
Preliminary studies suggest low survival and growth from pigfish larvae reared solely on rotifers and, therefore, a diet consisting entirely of copepod nauplii or fed in combination with rotifers is recommended (Ohs unpublished data). If fed in combination, a feeding regime that includes multiple feeding times per day should be implemented to ensure that larvae are continually feeding on nutritious rotifers. At a density of 50 fish larvae/L, it is suggested copepod nauplii be fed once a day, contained in the culture tank, at a density of 2–8 nauplii/mL. A copepod nauplii-rotifer combination should be fed four times a day at a density of 5–15 individuals/mL and allowed to flow out of the tank. This will ensure reduced foraging time for the fish larvae and keep the rotifer population and subsequent water quality problems from escalating at an uncontrollable rate. The density of live feed maintained should increase as fish larvae get larger and consume more. This regime should be followed until the larvae are capable of ingesting larger prey such as Artemia nauplii (Ohs unpublished data).
Little information is available on the procedures for feeding Artemia nauplii to pigfish larvae, but a density of 0.25–1.00 individuals/mL is recommended as a starting point. The culture tank should then be monitored and new Artemia added as the pigfish larvae clear the water (Stottrup and McEvoy 2003). As the Instar I stage (the initial stage) of Artemia is a non-feeding stage, it is incapable of enrichment. However, enrichments are suggested if subsequent stages are to be fed to pigfish larvae. Pigfish larvae reared at the IRREC were only fed the Instar I stage. Transitioning to an artificial diet begins shortly after the feeding of Artemia, but the dietary shift should be gradual to ensure a successful transition. Conversion to an artificial diet allows manipulation of nutrient intake of fish, eliminates live feed protocols, and reduces labor associated with maintaining live feeds.
Nursery
By 25 mm TL, the body of the pigfish has scales, all spines and fins are well-developed, and the overall appearance is that of an adult (Darcy 1983; Watson 1983). However, the deep body and characteristic pigmentation do not occur until the fish is fully formed, at approximately 70 mm TL (Darcy 1983). The developmental period in between is considered the juvenile phase or nursery phase in an aquaculture setting. Many diet studies of wild juvenile pigfish have been conducted (Carr and Adams 1973; Adams 1976; Howe 2001), but only limited information is available on the culture techniques for juvenile pigfish. Once the larvae have been successfully transitioned onto an artificial diet, transfer from the larval tank to a new system is essential. Ohs et al. (2009) explored growout techniques, discussed in detail in the growout section, which may also be applied to the nursery phase. Growth rates for wild juvenile pigfish during the first year of life range between 7.0–9.3 mm SL (standard length)/month from June through October to 3.1mm SL/month from October to April (Sutter and McIlwain 1987). An accelerated growth rate is likely to be attained under culture conditions.
Growout
The average size range for pigfish sold as bait is 76.0–152.0 mm (3–6 inches). Ohs et al. (2009) collected wild juveniles (67.0 mm and 5.40 g) and grew them in a recirculating aquaculture system consisting of (12) 85-liter aquaria for 65 days at three stocking densities; 0.1 fish/L, 0.3 fish/L, and 0.5 fish/L. Ambient water temperature and light cycle were maintained in a greenhouse and salinity was constant at 26.0 g/L. Pigfish were fed 3–7% of their biomass daily over two feedings with a 2.0 mm slow-sinking, pelleted commercial diet consisting of 50% crude protein and 15% crude lipid. During this study, juveniles grew 0.92–0.97 mm/day and 0.48–0.53 g/day. At this rate a 25.4 mm (1 inch) juvenile would take about 4 months to reach market size. Survival increased with an increase in stocking density (22–58%), but was quite variable. Feed conversion ratio was also variable ranging from 2.04 to 5.33, with a higher stocking density converting feed more efficiently. These preliminary data suggest that the rapid growth rate, quick conversion to an artificial feed, and improved survival warrant further studies in larger recirculating aquaculture systems to determine appropriate culture techniques and protocols.
Disease
Pigfish taken from Chesapeake Bay and off the coast of North Carolina were found to be parasitized by two species of monogeneans, Choricotyle aspinachorda and Pseudotagia cupida (Hendrix 1994). Both parasites infested the gill filaments of the pigfish. Other than this report, there are no disease issues described in the literature that are specific to pigfish.
On the contrary, pigfish appear to be a rather hardy species tolerating a wide range of environmental conditions; this makes them an ideal candidate for aquaculture, as well as use for live bait. However, their tolerance of extreme conditions outside of their environmental range is considered low and culture conditions outside of optimal ranges will likely result in reduced health and greater susceptibility to disease (Darcy 1983).
Market
In Florida, commercial and recreational landings of pigfish are not generally recorded. However, in 2005, landings of grunts totaled roughly 2.3 million pounds, 83% of which were caught by recreational anglers (FWRI 2016). On the gulf coast, 89% of those grunt landings consist of white grunt, Haemulon plumier, and pigfish (FWRI 2016). The majority of pigfish caught are either used as live bait or marketed for the live bait industry (Sutter and McIlwein 1987; FWRI 2016). The average retail price per fish as bait is $0.75–1.50, depending on size and supply during the time of year. Currently, there is no state law prohibiting or limiting the production of pigfish within the state of Florida because they are a species native to the region. Furthermore, competition is limited because marketing of non-native baitfish species is restricted and production protocols for other native baitfish species are not defined. The short time required to reach market size, close proximity to retail outlets, high consumer demand, and variable supply, make pigfish a strong candidate for Florida aquaculture.
Conclusion
Information pertaining to aquaculture techniques for pigfish is limited. However, the available information has increased in recent years, and initial studies indicate they lend themselves to captive production. In addition, pigfish are hardy, display a fast growth rate, and have a high market demand. All these aspects support current research and future interest in the development of pigfish aquaculture for the baitfish market.
References and Recommended Readings
Adams, S.M. 1976. Feeding ecology of eelgrass fish communities. Transactions of the American Fisheries Society 105: 514–519.
Carr, W.E.S. and C.A. Adams. 1973. Food habits of juvenile marine fishes occupying seagrass beds in the estuarine zone near Crystal River, Florida. Transactions of the American Fisheries Society 102: 511–540.
Collins, L.A. and J.H. Finucane. 1984. Ichthyoplankton survey of the estuarine and inshore waters of the Florida Everglades, May 1971 to February 1972. NOAA Technical Report NMFS 6 pp.
Darcy, G.H. 1983. Synopsis of biological data on the pigfish, Orthopristis chrysoptera (Pisces: Haemulidae). FAO Fisheries Synopsis No. 134. 23 pp.
FWRI (Fish and Wildlife Research Institute). 2016. Grunts. Florida Fish and Wildlife Conservation Commission. http://m.myfwc.com/media/4210230/20-grunts-2016.pdf
Hendrix, S.S. 1994. Marine flora and fauna of the eastern United States, Platyhelminthes: Monogenea. NOAA Technical Report NMFS 121. 112 pp.
Howe, J.C. 2001. Diet composition of juvenile pigfish, Orthopristis chrysoptera (Perciformes: Haemulidae), from the northern Gulf of Mexico. Gulf of Mexico Science 19(1): 55–60.
Lindeman, K.C. and C.S. Toxey. 2003. Haemulidae. Food and Agriculture Organization of the United Nations. https://www.fao.org/3/y4162e/y4162e18.pdf (August 2022).
Mata, E., J. Rosas, A. Velasquez, and T. Cabrera. 2004. Hormone to induce spawning and larval description of corocoro, Orthopristis ruber Cuvier (Pisces: Haemulidae). Journal of Marine Biology and Oceanography 39(1): 21–29.
Oesterling, M.J., C.M. Adams, and A.M. Lazur. 2004. Marine baitfish culture: workshop report on candidate species and considerations for commercial culture in the southeast U.S. Virginia Sea Grant Program, Marine Resource Advisory No. 77. 27 pp.
Ohs, C.L., S.W. Grabe, S.M. DeSantis, A.L. Rhyne, and M.A. DiMaggio. 2009. Successful culture of pinfish Lagodon rhomboides and pigfish Orthopristis chrysoptera in recirculating aquaculture systems at various stocking densities. Aquaculture America 2009 Abstracts p. 251.
Sutter, F.C. and T.D. McIlwain. 1987. Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (Gulf of Mexico) – pigfish. U.S. Fish and Wildlife Service Biological Report 82(11.71). U.S. Army Corps of Engineers, TR EL-82-4. 11 pp.
Stottrup, J.G. and L.A. McEvoy, eds. 2003. Live feeds in marine aquaculture. Blackwell Science Ltd. Oxford UK. 318 pp.
Warlen, S.M. and J.S. Burke. 1990. Immigration of larvae of fall/winter spawning marine fishes into a North Carolina estuary. Estuaries 13: 453–461.
Watson, W. 1983. Redescription of larvae of the pigfish, Orthopristis chrysoptera Linnaeus (Pisces, Haemulidae). U.S. Fish and Wildlife Service Fishery Bulletin 81: 847–854.