Gulf Killifish

Credit: MSU/Ag Communications Kat Lawrence
Introduction
Species selection in aquaculture is an extremely important first step towards a viable business. There is often limited information available to potential producers when considering culturing new species. Gulf killifish are a promising species for commercial aquaculture, but there are also limitations and culture system considerations of which producers need to be aware. This publication provides concise information to aid producers in making the most informed decision possible when considering Gulf killifish aquaculture.
General Description
The Gulf killifish, Fundulus grandis (Figure 1), is a member of the family Fundulidae, commonly referred to as topminnows. Topminnows are a large group of generally small-bodied fish of the order Cyprinodontiformes. They generally have an upturned mouth and dorsolaterally flattened head adapted to live near the top of the water column. Gulf killifish reach a maximum body length of about 5 inches (12.7 cm), making them a medium to large member of the family Fundulidae, most of which never get bigger than 4 inches (10.2 cm). An unpublished University of Florida IFAS survey identified the Gulf killifish and two related species as having the highest priority for development of an aquaculture-based live bait industry. Gulf killifish have distinctive superior or upturned mouths that allow them to feed efficiently on floating prey or diets. As adults, male Gulf killifish, as pictured on the left in Figure 1, generally have iridescent speckles on the sides of their bodies and a white or transparent strip on the distal (back) edge of an otherwise pigmented caudal (tail) fin. Breeding males will acquire a "beard," or darkening of the underside of the head and gill plates. Females, as depicted on the right in Figure 1, maintain a drab olive-brown color throughout the year with no speckles.
Geographic Distribution and Habitat
Gulf killifish are found in coastal areas from Flagler Beach in northeastern Florida south and west, throughout the Gulf of Mexico, to Veracruz, Mexico. They are common in brackish-water Spartina marshes and mangrove areas. Around barrier islands, Gulf killifish are more common on the bay side than the ocean side. This species tolerates a wide range of salinities (euryhaline) and is known to occur anywhere from hyper-saline (> 35 ppt) tide pools to near freshwater in coastal streams and rivers. Gulf killifish do not venture very far into open water, away from the protection from predation provided by marsh vegetation.
Natural History
Gulf killifish generally are found in brackish salt marsh estuaries. They have been shown to move into the intertidal zone on the flood tide, where they feed mostly on small crustaceans, snails, and worms and exit back to subtidal areas on the ebb tide (Rozas and LaSalle 1990). It is rare to find a wild killifish more than 2 years old, although broodstock held in captivity have been observed to reach age 4. Gulf killifish can reach sexual maturity by the end of their first year, and spawn over an extended period from spring to fall depending on latitude. Peaks in egg production during the spawning season are tied to lunar cycles, as this species naturally concentrates spawning during the two highest tides of the month. Females have relatively low fecundity, with each individual laying a few hundred eggs attached to the vegetation near the high tide line. When the tide falls, embryos are able to complete development out of water (a beneficial characteristic for producers, discussed below) until the next high tide inundates the eggs and induces hatching. Gulf killifish larvae hatch with fully functional eyes and mouths (another beneficial characteristic for producers) and are able to start searching for and consuming food immediately. As a small baitfish, this species is preyed upon by many other fishes including many of the game fish species they are used to target such as speckled trout, redfish, and flounder.
Culture Techniques
The culture techniques for this species have been extensively studied over the last several decades. Traditionally, culture of Gulf killifish has relied on a three-phase brackish water pond system. This consisted of a system of broodstock ponds, hatching ponds, and grow-out ponds (Trimble et al. 1981; Perschbacher and Strawn 1985; Tatum 1982; Waas et al. 1983; Wallace and Waters 2004). These pond culture methods, however, have not gained much commercial interest largely due to the limited availability and high cost of land with access to saline water. Recent research, the majority of which is described in Anderson et al. (2012) and Green (2013), has resulted in new culture techniques that may be of benefit to those interested in producing this species. These newer culture procedures have incorporated an indoor hatchery phase, where eggs are incubated and hatched (Figure 2). The current article will summarize the earlier information and add results of recent research and their implications on production techniques.
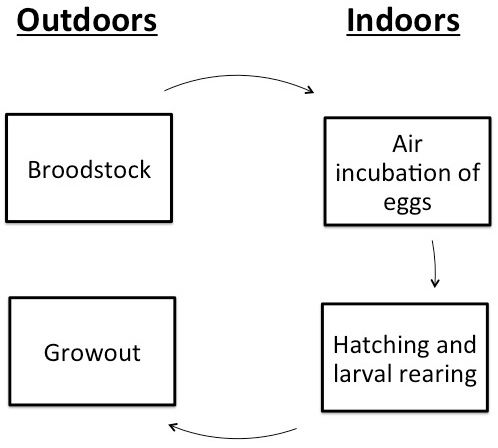
Broodstock
Aquaculture methods for Gulf killifish are relatively well-developed. Broodstock can be held and allowed to spawn in ponds or outdoor pools. No hormone injection is required to induce spawning in this species. However, because innately low fecundity is problematic for producing large numbers of fish, optimizing broodstock performance is critical. Research indicates that maximum egg production is obtained from females at least 4 inches in length (Patterson et al. 2013). Outdoors, spawning can be expected to begin as water temperatures reach 68°F (20°C). Aquaculture broodstock of a variety of species are generally fed more expensive, high quality diets because broodstock diets can affect fecundity and egg quality. Studies have been conducted to test the effects of broodstock diet on egg and larval quality for Gulf killifish. Results showed that diets containing cheaper vegetable oils and plant proteins as a total substitute for expensive ingredients such as fishmeal and fish oil did not affect overall egg production, fertilization, or larval fitness in a meaningful way (Patterson and Green 2014). These findings in Gulf killifish indicate that producers can use less costly feed with less fishmeal inclusion, thus reducing the expense of maintaining broodstock.
Egg Collection and Incubation
Eggs are collected from Spawntex (Blocksom & Co., Michigan City, IN, USA) spawning mats that are suspended horizontally 8–10 in (20–25 cm) below the broodstock pond surface. Eggs are mildly adhesive but lose most of their adhesiveness 24 hours after they are spawned. Eggs can then be removed from the spawning mats by shaking or beating the mats over a tub filled with a few inches or water. After removal from the mat, eggs are rinsed and cleaned over a 1–1.5 mm screen and enumerated volumetrically (approximately 114 eggs/mL) (Green et al. 2010; Ramee 2015). The percent fertilization of the eggs can also be easily assessed at this time. When first collected, eggs will appear clear with a brown/yellow tint. As they develop, dead or unfertilized eggs will turn white and opaque, while healthy eggs will first look as if they have been sprinkled with pepper (appearance of chromatophores or pigment-containing cells) (Figure 3). Following this stage, eyes and eventually the embryo's beating heart will become visible through the clear outer membrane.
In the past, eggs have been transferred directly to the hatching pond on the spawning mats. Though this technique requires less labor, it does not allow the producer to observe developing eggs or control environmental conditions. There are also concerns with decreased larval survival in ponds due to predation. The biggest issue, however, is that killifish eggs incubated in water hatch over an extended period, which can lead to uneven size distribution and cannibalism within the cohort. Air incubation is preferred because it allows the producer to hatch the entire cohort of eggs at once.
Air incubating Gulf killifish eggs is a relatively simple process. Once the eggs are consolidated and cleaned, they can be spread thinly between two pieces of non-toxic foam (such as Polly-Fil True-foam, Fairfield, Danbury, CT) inside a plastic container. This technique is described in detail by Anderson et al. (2012). If temperature control is desired, an incubator can be made out of a retrofitted refrigerator with an external thermostat. Temperature control can be used to synchronize the hatch of multiple spawns of eggs into a single large cohort of fry. Egg development takes longer at lower temperatures so, by adjusting the incubation temperature of different batches of eggs, they can be hatched simultaneously. For example, eggs collected one week could be incubated at 68°F (20°C) for 18 days, and eggs collected one week later could be incubated at 79°F (26°C) for 11 days. Both batches could then be hatched together and effectively double the size of that production cycle. Air incubation containers should be moistened with saline water (5–18 ppt (g/L)) in order to improve embryo survival and hatch rate. This salinity helps reduce fungal and mold growth on the eggs. The highest hatch rates (70–80%) have been observed at 7–7.5 ppt salinity (Coulon et al. 2012; Brown et al. 2012). Once the embryos are submerged after incubation, a producer can expect 90% hatch within 2 hours and 100% hatch within 24 hours.

Credit: Shane Ramee, UF/IFAS
Hatchery
Hatching and larval rearing of Gulf Killifish may benefit from being conducted in indoor tank systems as opposed to the traditional second phase hatching and larval pond. This could be done with either recirculating or flow through saline (5–12 ppt) water systems. An indoor setting reduces predation risk and increases environmental control. Also, if a producer has limited saltwater access, indoor recirculating systems allow for the most efficient use of this resource.
Studies have shown that killifish benefit from a saline environment in their early life stages but their freshwater tolerance improves as they grow and develop. Larval survival to 2 weeks old was reduced by direct transfer into freshwater (0 ppt) at hatch after air incubation at 7 ppt (Ramee 2015). Direct transfer of 2-week-old larvae from 7.5 ppt to freshwater reduced fry growth and survival over the next four weeks compared to transfers into 2.5, 5.0, and 7.5 ppt (Ramee et al. 2016). However, transfer of 7-week-old fish from 7.5 ppt to freshwater resulted in no difference in survival and growth compared to controls (Ramee et al. 2016). This same result was found when the transfer experiment was repeated with 12-week-old fish (Ramee et al. 2016). On the other hand, another study found juvenile (~ 8-month-old) Gulf killifish growth and survival were lower in freshwater over a 12-week trial compared to 8 and 12 ppt (Patterson et al. 2012). Though there may be some differences in freshwater tolerance between different populations, Gulf killifish should remain in a saline environment for at least 7 weeks before stocking for grow-out.
Feeding of Gulf killifish should begin the day of hatch. They are capable of consuming Artemia nauplii soon after hatch. Refer to Treece (2000) for information on hatching Artemia cysts. Feeding rates should be increased daily, and there should always be live Artemia nauplii available during daylight hours. New research has shown that Gulf killifish can survive and grow when exclusively fed a commercially available prepared diet from hatch. Many larval diets are available, which are designed to supplement or replace live feeds. Multiple diets have been successfully used from hatch in experimental trials while others produced less promising results (Patterson et al. unpublished). While live Artemia led to the best growth and survival, commercial larval diets are less expensive, eliminate extra labor required to hatch Artemia, and reduce the possibility of disease transfer associated with live feeds. Success through the larval stage is dependent on many factors but good survival from hatching can be obtained with live feeds (~85%), prepared larval diets (~70%), or a combination of the two (Patterson et al. unpublished). Dependent on growth rate, transition from larval to juvenile takes about three weeks, with the first appearance of scales indicating a juvenile fish.
Grow-Out
Due to their euryhaline nature, Gulf killifish can be grown to market size in fresh, brackish, or saltwater. Broodstock have been held in freshwater research ponds at Mississippi State University for over 3 years. A recent study has suggested that by 7 weeks post-hatch, juvenile killifish are completely tolerant to freshwater and grow and survive as well as they do in brackish (7.5 ppt) water (Ramee et al. 2016). This means that these fish can be stocked directly from their saline hatchery tanks into freshwater ponds for grow out at 7 weeks post-hatch. Depending on production goals, several cohorts of fish from the hatchery may be combined and graded before stocking into ponds. Outdoor ponds or pools are preferred over indoor tanks because the natural productivity serves as an additional nutrition source.
Growth is density-dependent, and stocking density should reflect the desired growth rate and time to market. According to Wallace and Waters (2004), stocking 0.3- to 0.5-g fish at 50,000/acre (123,500/ha) and 100,000/acre (247,000/ha) results in harvest-size fish 2.5 inches (64 mm) in 5 weeks and 6.5 weeks, respectively. At 200,000/acre, the fish barely grow at all. This information can be used to time the harvest of the minnows when demand is high.
Though the natural productivity of the ponds does provide some nutrition, the availability can be inconsistent and is dependent on the fertilization regime, season, and stocking density of the pond. For this reason, supplemental feeding is recommended, even if it only acts as an additional source of fertilization. In ponds it is acceptable to use less expensive, lower-quality feed during pond grow-out. A <1 mm floating pellet with <35% protein and <5% fat has proven to be an acceptable diet. Pellet size should be matched relative to fish mouth gape size in order to ensure efficient feeding. Fish can be fed 10% body weight per day for the first 2 weeks after stocking. Afterwards, a subsample should be weighed, and the feeding rate adjusted to 5% body weight per day. When fish near market size, feeding rate can be further reduced to 3% body weight per day.
Market and Economics
According to a survey of Florida saltwater anglers and bait dealers conducted from 2011–2013, Fundulus spp. are the second most popular near-shore species sold among bait dealers and the fifth most popular baitfish species among anglers (DiMaggio and Ohs unpublished). An economic analysis of Gulf killifish culture in Florida found that all bait dealers are currently reliant on wild-caught supply, and killifish demand exceeds overall supply (Adams and Lazur 2001). The demand and price of killifish is also highly seasonal and varies by location, corresponding to specific game fish targeted. There are seasonal demands for small Gulf killifish in the winter for flounder anglers and larger individuals for multiple other species year-round. The Adams and Lazur analysis assumed fish were sold into the wholesale market for $0.07 each. Finally, they concluded that both a 5- and 10-acre (2- and 4-ha) pond-based production system would be profitable for the producer. Another economic analysis by Anderson et al. (2012) found a 10-acre (4-ha) pool spawning and pond grow-out system to be the most profitable. This analysis assumed direct sale to a bait shop or individual anglers at $0.15 per fish. Both economic analyses subdivided the water-acreage into ponds of 0.5 acre (0.2 ha) or less. Neither of these analyses considers an inland facility utilizing an indoor hatchery and outdoor freshwater grow-out. This style of production system deserves a formal economic analysis in the future because it may have applicability for current Florida pond aquaculture producers. Florida has several locations with low-salinity groundwater, which could be ideal for killifish culture, similar to areas in Alabama (Phelps et al. 2010).
The Gulf killifish is native to the Atlantic and Gulf of Mexico coasts of Florida and all coastal states of the Gulf. Thus, there are no special laws or regulations for culturing them in Florida other than attaining an Aquaculture Certificate from Florida Department of Agriculture and Consumer Services Division of Aquaculture. If you plan on transporting them to other states, check the laws of those states.
Conclusion
The Gulf killifish is a strong candidate for commercial aquaculture production in Florida. Main impediments to culture are low fecundity and relatively low market price per fish. The methods for culturing this species have improved in the past decade, and the new methods described in this paper allow for greater control of reproduction, larval growth, and survival. Additionally, the markets for Gulf killifish are strong especially in regions with estuarine and inshore fishing locations. Gulf killifish have the potential to help diversify the marine baitfish aquaculture industry in Florida and throughout the southeastern United States.
References
Adams, C., and A. Lazur. 2001. Economic considerations for the prospective mudminnow culturist in Florida. FE309. Gainesville: University of Florida Institute of Food and Agricultural Sciences. http://ufdc.ufl.edu/IR00001920/00001
Anderson, J. A., C. C. Green, J. Christoferson, and J. Patterson. 2012. Cocahoe Minnow Production Manual. Sea Grant Louisiana, Baton Rouge, LA. http://www.lsuagcenter.com/en/crops_livestock/aquaculture/baitfish/minnows/Cocahoe-Minnow-Production-.htm.
Brown, C. A., F. Galvez, and C. C. Green. 2012. "Embronic development and metabolic costs in Gulf killifish Fundulus grandis exposed to varying environmental salinities." Fish Physiology and Biochemistry 38(4):1071–82.
Coulon, M. P., C. T. Gothreaux, and C. C. Green. 2012. "Influence of substrate and salinity on air-incubated Gulf killifish embryos." North American Journal of Aquaculture 74(1):54–59.
Green, C. 2013. Intensive (Non-pond) Culture of Gulf Killifish. Southern Regional Aquaculture Center. Publication 1202. https://srac.tamu.edu/fact-sheets/serve/267
Green, C., C. Gothreaux, and C. Lutz. 2010. "Reproductive output of Gulf killifish at different stocking densities in static outdoor tanks." North American Journal of Aquaculture 72(4):321–331.
Patterson, J., C. Bodinier, and C. Green. 2012. "Effects of low salinity media on growth, condition, and gill ion transporter expression in juvenile Gulf killifish, Funulus grandis." Comparative Biochemistry and Physiology, Part A 161(4):415–21.
Patterson, J., C. Ohs, A. Palau, P. O'Malley, L. D'Abramo, R. Reigh, and C. Green. unpublished. "Feeding larval Gulf killifish Fundulus grandis: Live diet replacement at first feeding and utilizing a transitional feeding regime of live and artificial diets."
Patterson, J. T., T. G. Allgood, and C. C. Green. 2013. "Intraspecific variation in reproductive potential with maternal body size in Gulf killifish Fundulus grandis." Aquaculture 384:134–139
Patterson, J. T., and C. C. Green. 2014. "Physiological and reproductive response to varying quantitative lipid inclusion in diets for Gulf killifish Fundulus grandis Baird and Girard." Aquaculture Research 49(9):2236–2247.
Perschbacher, P. W., and K. Strawn. 1985. "Fertilization vs. feeding for growout of pond-raised Gulf killifish." Proceedings of the Annual Conference of the Southeastern Association of Game and Fish Commissioners, 335–342.
Phelps, R. P., W. H. Daniels, N. R. Sansing, and T. W. Brown. 2010. "Production of Gulf killifish in the Black Belt region of Alabama using saline groundwater." North American Journal of Aquaculture 72(3):219–224.
Ramee, S. W. 2015. Low salinity tolerance of Gulf killifish Fundulus grandis with relavence to aquaculture. Theses and Dissertations. 2978. https://scholarsjunction.msstate.edu/td/2978 [Updated 5/2/2022]
Ramee, S. W., C. Green, and P. J. Allen. 2016. "Effects of Low Salinities on Osmoregulation, Growth, and Survival in Three Age Groups of Juvenile Gulf Killifish Fundulus grandis." North American Journal of Aquaculture 78, 8–19.
Rozas, L. P., and M. W. LaSalle. 1990. "A comparison of the diets of Gulf killifish, Fundulus grandis Baird and Girard, entering and leaving a Mississippi brackish marsh." Estuaries 13(3):332-336.
Tatum, W. M. 1982. "Production of bull minnows (Fundulus grandis) for the live bait market in coastal Alabama." Alabama Marine Resources Laboratory, Marine Resources Division, Department of Conservation and Natural Resources.
Treece, G. D. 2000. "Artemia production for marine larval fish culture." Southern Regional Aquaculture Center. Publication 702. https://srac.tamu.edu/fact-sheets/serve/142 [Updated 5/2/2022]
Trimble, W. C., W. M. Tatum, and S. A. Styron. 1981. "Pond studies on Gulf killifish (Fundulus grandis) mariculture." Journal of the World Mariculture Society 12(2):50–60.
Waas, B. P., K. Strawn, M. Johns, and W. Griffin. 1983. "The commercial production of mudminnows (Fundulus grandis) for live bait: a preliminary economic analysis." Texas Journal of Science 35(1):51–60
Wallace, R. K., and P. L. Waters. 2004. "Growing bull minnows for bait." Southern Regional Aquaculture Center. Publication 1200. https://srac.tamu.edu/fact-sheets/serve/149