Overview
In 2018, the total value of aquacultured ornamental fish sold by farmers in the United States was estimated to be $41.3 million, with marine ornamental fish sales contributing $8.9 million (USDA-NASS 2019). Florida is the leading producer of aquacultured ornamental fish in the United States with $28.7 million in sales in 2018 (USDA-NASS 2019).
Marine ornamental fish fetch a much higher price per unit than their freshwater counterparts. Despite the higher value of marine fish, far fewer farms produce them in the United States than produce freshwater ornamental fish. A 2018 estimate counted only 28 marine ornamental fish farms in the United States, 15 of which were located in Florida. In that same year, farms producing freshwater ornamentals numbered 204 (USDA-NASS 2014).
Freshwater ornamental aquaculture dominates the industry, with over 90% of freshwater aquarium fish available as captive bred, while the vast majority of marine ornamentals are wild captured (Dawes 1999). Yet, this small sector of the aquaculture industry has grown substantially in recent years. Initially, only clownfish, dottybacks, and gobies were commercially available as captive bred. According to Coral Magazine's Captive-Bred Marine Fish Species List for 2019, about 398 species of marine ornamental fish have been successfully bred in captivity, and approximately 46 of those species are considered commonly available.
Most commercial facilities producing marine ornamental fish are near the ocean for access to salt water. Intensive culture in recirculating aquaculture systems is most often used to allow complete control over water parameters, conserve water usage, and maximize production. Facilities normally produce numerous varieties of clownfish, a staple species, alongside additional species from several families.
Life history traits characterizing most commercially produced marine ornamental species include monogamy, demersal spawning, and extended parental care. The resulting larvae of species with these traits are usually precocial, meaning they are well-developed at hatching and have a higher rate of survival. Precocial larvae also tend to be slightly larger and able to consume and digest larger prey items such as rotifers and newly hatched Artemia as first-feed items.
Pelagic spawning species (those that spawn small, positively buoyant eggs) often produce altricial larvae. Altricial larvae are under-developed at hatching and usually require smaller live zooplankton (copepods or ciliates) at first feeding. These species still face significant technological bottlenecks that make them more difficult to produce economically. They are therefore underrepresented on the current list of captive-bred fish.
This publication briefly reviews the more common groups of marine ornamental fishes cultured in the United States. For simplicity, fish are grouped according to family in order of general prevalence in the marine ornamental aquaculture industry, and a representative photograph is included for reference.
Clownfishes and Damselfishes (Pomacentridae)
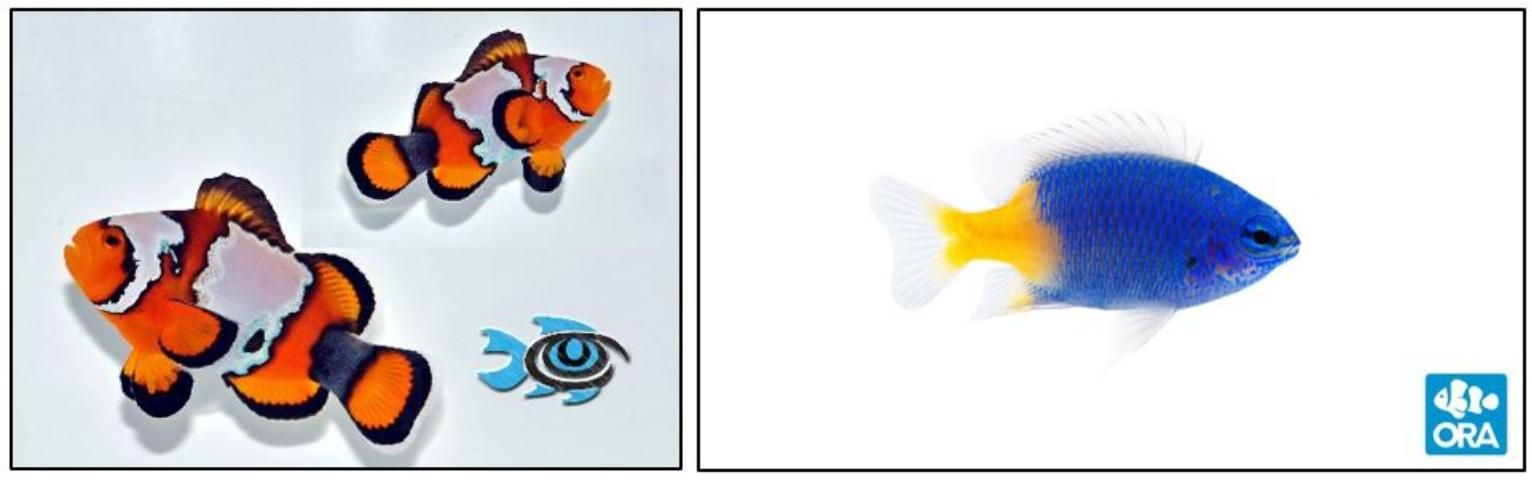
Credit: FishEye Aquaculture (Dade City, FL) and Oceans Reefs and Aquariums (ORA) (Ft. Pierce, FL).
Clownfish, anemonefish, and damselfish belong to the family Pomacentridae and are found in most tropical seas concentrated among shallow coastal reefs, with the greatest diversity in the Indo-Pacific region (Thresher 1984). Damselfish and clownfish display unique behaviors. Clownfish often form a symbiotic relationship with anemones whereby the fish are protected from predators. The fish take shelter within the stinging tentacles, to which they are resistant, and in return the fish provide the anemone with food. Additionally, clownfish are monogamous protandrous hermaphrodites, meaning they form mating pairs and can change sex from male to female, with the female often being larger and more dominant. Damselfish and clownfish are small and brightly colored, making them popular additions to home and public aquaria.
With their bright coloration, small size, and hardiness, Pomacentrid species comprise over half of all marine ornamental fish imported into the United States for the aquarium industry (Rhyne 2012). Additionally, ten out of the top 20 most imported species belong to this family, collectively adding up to millions of fish imported yearly (Rhyne 2012). Pomacentrid genera important to the aquarium industry include damselfish (Chrysiptera and Dascyllus), chromis (Chromis), and clownfish (Amphiprion and Premnas).
While over 30 species of damselfish have been successfully bred and raised in captivity, very few are commercially aquacultured. Although damselfish are demersal spawners with moderate parental care, newly hatched damselfish larvae are considered altricial and often require small first-feed items like copepod nauplii (Gopakumar and Santhosh 2009). This makes production of many damselfish species not economically feasible when compared to the price of their wild-caught counterparts.
Conversely, clownfish and their numerous color morphs are widely considered a staple species for marine ornamental aquaculture operations. Broodstock pairs are often created by choosing one small and one large fish with the intention of the former being male and the latter being female (Hoff 1996). Once successfully paired, they will form a monogamous bond and routinely spawn bi-monthly if properly conditioned (Hoff 1996). The female will lay adhesive, elliptical eggs on a substrate, often a tile or clay pot (Moe 1982/1992). The male will follow close behind and fertilize the eggs, subsequently guarding and cleaning the nest until hatching (Moe 1982/1992). Clownfish larvae are precocial and easily take enriched rotifers as a first-feed item and newly hatched Artemia about a week later (Hoff 1996). Settlement averages between 10 to 20 days post hatch (DPH) and is dependent upon numerous variables including species and culture environment (Moe 1982/1992). For more detailed information concerning clownfish culture, please refer to SRAC publication #7213 "Species Profile: Clownfish" (DiMaggio et al. 2017).
Dottybacks (Pseudochromidae)

Credit: Proaquatix (Vero Beach, FL)
Mainly found in the Indo-West Pacific, dottybacks are a significantly smaller family compared to Pomacentridae (Madhu et al. 2016). They typically consume small invertebrates and are characterized by an elongated body and interrupted lateral line (Thresher 1984). Dottybacks are small, brightly colored, and hardy, making them perfect additions to any marine aquarium.
To date, about 28 Pseudochromidae species have been successfully captive bred. Common dottyback species produced in marine ornamental aquaculture include the orchid dottyback (Pseudochromis fridmani), orange or neon dottyback (Pseudochromis aldabraensis), and splendid dottyback (Manonichthys splendens).
Most members of the family Pseudochromidae are protogynous hermaphrodites, beginning life as a female and having the ability to transition to the male sex later in life. Some Pseudochromidae even exhibit bi-directional sex change in laboratory settings, where the fish may transition back and forth between sexes (Wittenrich and Munday 2005). Depending on species, the male is often larger and sometimes more colorful than the female. In production facilities, dottybacks are normally housed in pairs (Olivotto et al. 2006). They are demersal spawners which means they spawn large, negatively buoyant eggs. In their natural habitat, females lay a ball of eggs held together by sticky threads inside a crevice. Producers usually provide small-diameter PVC pipe to mimic these crevices in a production setting (Madhu et al. 2016). The male will guard the eggs until the larvae hatch. Newly hatched larvae are precocial and can immediately consume enriched rotifers as a first-feed item and newly hatched Artemia less than two weeks later (Madhu et al. 2016). Larvae generally initiate metamorphosis between 25 and 35 DPH (Olivotto et al. 2006).
Gobies (Gobiidae)

Credit: Oceans Reefs and Aquariums (ORA) (Ft. Pierce, FL)
The family Gobiidae, the largest family of marine fishes, are found in most tropical and subtropical seas with some species inhabiting fresh or brackish water (Thresher 1984). Gobies are generally small, less than four inches (10 cm), and benthic with most possessing a fused pelvic fin that can function like a suction disk (Thresher 1984).
Gobies are the fourth most imported family of marine ornamental fishes into the United States, demonstrating their high demand in the aquarium trade (Rhyne 2012). To date, about 49 species have been captive bred, many belonging to genera Elacatinus, commonly known as neon or cleaner gobies, and Cryptocentrus, or watchman gobies.
Some gobies form symbiotic relationships with pistol shrimp. These so called "shrimp gobies" belong to the genera Amblyeleotris, Cryptocentrus, and Stonogobiops, and are very popular in aquariums due to this unique behavior (Thacker et al. 2011). The yellow prawn-goby (Cryptocentrus cinctus) is a commonly aquacultured shrimp goby.
Perhaps the most produced genus in the goby family is Elacatinus, which is almost exclusively found in the Western Atlantic (Sazima et al. 2000). These fish are very small, less than two inches (5 cm), and often form symbiotic relationships with larger predatory fish by providing a cleaning service in exchange for food in the form of external parasites (Sazima et al. 2000). Commonly cultured species in this genus include the neon goby (E. oceanops), barber goby or yellowline goby (E. figaro), and sharknose goby (E. evelynae).
In general, gobies are straightforward to breed and raise in captivity. Depending on species, they will spawn in pairs or small harems, often choosing the interior of small-diameter PVC pipe as a spawning surface. Being demersal spawners with high paternal parental care, newly hatched larvae are often precocial with a mouth gape large enough to take enriched rotifers as a first-feed item. Time to metamorphosis is highly species dependent.
Blennies (Blenniidae)

Credit: Proaquatix (Vero Beach, FL)
The family Blenniidae contains less than half the number of species found in the Gobiidae family but is no less diverse with respect to colors, behaviors, etc. (Thresher 1984). Blennies inhabit the reefs of the Atlantic, Pacific, and Indian Oceans. Some, however, can be found in brackish and freshwater environments (Thresher 1984). Most blennies are demersal and lack swim bladders, but there are a few genera, such as the fang blennies (Meiacanthus), that do have a functional swim bladder and reside at mid water column (Smith-Vaniz 2007). True to their name, fang blennies have two enlarged canines on their lower jaws that contain venom and are used for defending themselves and their territories (Smith and Wheeler 2006). Care should be taken when handling these fish, although bites to humans are rare.
Almost all blennies produced in captivity are species from the genus Meiacanthus, including the striped fang blenny (M. grammistes), blackline fangblenny (M. nigrolineatus), and the Mozambique fangblenny or harptail blenny (M. mossambicus). The remainder of this section will pertain to blenny species in the Meiacanthus genus.
Fang blennies are demersal spawners with high parental care exhibited by the male (Moorhead and Zeng 2011). Sections of small-diameter PVC pipe (1-inch) make appropriate nest sites (Olivotto et al. 2010). Harems are generally more productive than pairs because several females may lay multiple small batches of eggs in the same nest area (Moorhead and Zeng 2011). Lacking sexual dimorphism, adults are difficult to sex. It is good practice to start with a large group and let pairs or harems naturally form. Newly hatched larvae are precocial and will thrive on enriched rotifers as a first-feed item. Time to settlement is approximately 35 DPH (Olivotto et al. 2010).
Cardinalfishes (Apogonidae)

Credit: Oceans Reefs and Aquariums (ORA) (Ft. Pierce, FL)
The family Apogonidae are easily recognized by their large mouths and eyes. Like Pomacentridae, Apogonidae contains many species (Thresher 1984). They are native to the Atlantic, Pacific, and Indian Oceans, primarily in marine and brackish water environments with a few species inhabiting freshwater (Thresher 1984).
Small body size, bright coloration, and hardiness make cardinalfish excellent aquarium residents. This family is the sixth most imported family of marine ornamental fish into the United States by volume (Rhyne 2012).
Perhaps the most well-known member of the family is the Banggai cardinalfish. This fish has a geographic range of less than 10,000 km2, which is limited to the mangrove coastlines of the Banggai Archipelago in Indonesia (Vagelli 2004). This species faced severe population declines in the late 1990s due to overharvesting for the world aquarium trade (Vagelli 2004). Fortunately, the need for conservation efforts was realized in the early 2000s and the Banggai cardinalfish population rebounded, in part due to increased aquaculture efforts (Hopkins and Tamaru 2005).
To date, around 16 species of cardinalfish have been successfully aquacultured with the most commonly produced species being the Banggai cardinalfish (Pterapogon kauderni) and the pajama cardinalfish (Sphaeramia nematoptera).
It is generally thought that keeping cardinalfish in pairs is best for production purposes; however, it can be difficult to establish this 1M:1F ratio due to the lack of distinctive sexual dimorphism (Hopkins and Tamaru 2005). The only indication of the male sex is a squared-off jaw line, but this isn't always apparent (Hopkins and Tamaru 2005). The only definitive way to identify a male is to wait until the fish spawn; cardinalfish are paternal mouthbrooders, and an enlarged mouth full of eggs is easily recognized. The male holds the eggs in his mouth while they incubate and will fast during this time (Hopkins and Tamaru 2005). In their natural environment, cardinalfish larvae will hatch and be retained within the mouth of the male for about one week before being released (Vagelli 2004). In an aquaculture setting, the egg mass is usually removed just before hatching to avoid paternal cannibalism (Hopkins and Tamaru 2005). The newly hatched larvae are precocial and will readily take enriched rotifers as a first-feed item (Hopkins and Tamaru 2005).
Seahorses (Syngnathidae)

Credit: Proaquatix (Vero Beach, FL)
Just under 300 species make up this unique family of fishes that include seahorses, pipefishes, and seadragons (Thresher 1984). The greatest numbers are found in warm temperate to tropical shallow marine environments of the Atlantic, Pacific, and Indian Oceans (Thresher 1984). Their narrow mouths enable them to use suction feeding to capture small invertebrate prey items. Both pipefishes and seahorses are popular in the marine aquarium trade, but the latter are more commonly produced in the aquaculture industry and will be the focus of the remainder of this section.
Seahorses (genus Hippocampus) are easily recognized by their upright orientation, prehensile tails, and elongated snouts. Despite their bony, tough looking exteriors, these delicate fish have limited mobility and have historically experienced population declines because of habitat destruction, bycatch, and overexploitation for the aquarium trade and traditional Chinese medicine (Koldewey and Martin-Smith 2010). Seahorse aquaculture production has increased in recent years in part to help alleviate anthropogenic impacts on wild populations (Lin 2008).
About 28 species of seahorse have been captive bred with about five species currently being commercially produced, including the lined seahorse (H. erectus) and spotted or yellow seahorse (species complex H. kuda). Seahorse pairs are normally monogamous and display complex mating rituals (Lin 2008). Male Hippocampus have a unique brood pouch into which the female deposits her eggs. The male will carry the incubating eggs until they hatch within the pouch and then release them into the water column. The fast-growing young are relatively large and well developed, able to consume newly hatched Artemia a few hours after birth (Lin 2008).
Tangs (Acanthuridae)

Credit: Top: Adrian Pingstone, Wikimedia Commons; Bottom: Tyler Jones, UF/IFAS
An exclusively marine and rather small family of ornamental fishes, Acanthuridae includes the tangs, surgeonfish, and unicornfishes with only five species found in the Atlantic and the rest (approximately 80) in the Pacific and Indian Oceans (Thresher 1984). They are primarily herbivorous and have small mouths with teeth used to scrape algae from surfaces (Thresher 1984). Acanthurids are characterized by a sharp spine located on both sides of the caudal peduncle (Thresher 1984).
Acanthuridae is the fifth most imported family of marine ornamental fish into the United States (Rhyne 2012). To date, only eight tang species in the family Acanthuridae have been captive bred.
In 2015, Dr. Chatham Callan at the University of Hawaii Oceanic Institute successfully produced captive-bred yellow tangs (Zebrasoma flavescens), which was a first for the Acanthuridae family. Research conducted at the Oceanic Institute continues to improve yellow tang commercial production.
About a year after the yellow tang breakthrough, the first batch of captive-bred blue tangs (Paracanthurus hepatus) was produced at the University of Florida Tropical Aquaculture Lab by adapting yellow tang larval rearing methods (DiMaggio et al. 2017). Small batches of blue tang have since been produced; however, several culture bottlenecks need to be examined before commercial production of this species will be feasible. Convict tangs Acanthurus triostegus, purple tangs Zebrasoma xanthurum, orangebar surgeonfish Acanthurus olivaceus, and yellowfin surgeonfish Acanthurus xanthopterus have also been recently raised on a small scale by commercial aquaculture producers, demonstrating the growing potential for captive propagation of this family of fishes.
Tangs are pelagic spawners with altricial larvae that require small first-feed items such as copepod nauplii (DiMaggio et al. 2017). Initiation of metamorphosis has been recorded to occur as early as 50 DPH (DiMaggio et al. 2017). It is important to note that members of this family are highly sensitive and prone to disease. Optimal water quality, nutrition, and general husbandry are critical in maintaining healthy and productive broodstock.
Wrasses (Labridae)

Credit: Matthew DiMaggio, UF/IFAS
The second largest family of marine ornamental fishes is the wrasses (Thresher 1984). Wrasses are exclusively marine, often inhabiting shallow tropical and subtropical coral reef ecosystems in the Atlantic, Pacific, and Indian Oceans, with very few species found in temperate waters such as the ballan wrasse (Labrus bergylta) (Thresher 1984).
Protractible mouths allow these carnivorous fish to constantly graze on various invertebrates, with some wrasse species providing a cleaning service to larger fishes by removing dead skin, mucus, and external parasites (Bshary and Schäffer 2002). Labrids are highly diverse in color and are generally small, making many species suitable for aquaria.
Most wrasses are protogynous hermaphrodites, meaning they can change sex from female to male, and have complex haremic mating systems. Typical spawning harems consist of several females (initial phase) and one dominant male (terminal phase), sometimes with additional younger males (intermediate phase). Males and females within harems broadcast spawn in small groups or pairs, releasing small, buoyant, pelagic eggs into the water column with no subsequent parental care.
Wrasses are also the second most imported family of marine ornamental fishes into the United States, making them some of the most popular fishes in the marine aquarium trade (Rhyne 2012). Despite this fact, there is currently no commercial production of any ornamental wrasse species, meaning that all specimens available in the trade are wild caught. To date, less than 10 species have been successfully bred and raised in captivity. This is due to many bottlenecks such as the complexity and fluidity of broodstock harems and the delicate altricial larvae of these species that require very small live prey such as copepod nauplii. The larval phase of wrasses is highly variable among species and ranges from 15 to 121 DPH (Victor 1986). Many wrasses in the genus Halichoeres have relatively short larval durations averaging between 20 to 30 DPH (Victor 1986) and are popular in the aquarium trade, making them ideal candidates for commercial production. Research at the University of Florida Tropical Aquaculture Laboratory is currently focusing on developing production protocols for several species of Halichoeres wrasses (H. melanurus, H. chrysus, and H. iridis), laying the foundation for the commercialization of wrasse aquaculture (Groover 2018).
Conclusion
The eight families described in this publication represent the majority of marine ornamental fish produced for the aquarium industry. There are many other species from various families that are produced in smaller numbers and with less frequency such as marine angelfishes (Pomacanthidae), dragonets (Callionymidae), and filefishes (Monacanthidae). Most marine ornamental fish found in the aquarium trade are still wild caught, but the variety and availability of captive-bred marine species is growing. Currently, demersal-spawning species with precocial larvae dominate the industry. As larval rearing, live-feeds-production technologies, and general life history knowledge continue to improve, it is possible that increased numbers of pelagic-spawning species will become economically feasible to produce and begin to supplement wild-caught stocks in the marine aquarium trade.
References
Bshary, R. and D. Schäffer. 2002. "Choosy Reef Fish Select Cleaner Fish that Provide High-Quality Service." Animal Behaviour 63(3): 557–564.
Dawes, J. 1999. "International Experience in Ornamental Marine Species Management – Part 2: Some Resource Management Strategies." Ornamental Fish International Journal 27: 10–12.
DiMaggio, M. A., E. M. Groover, J. van Senten, and M. Schwarz. 2017. "Species Profile: Clownfish." USDA Southern Regional Aquaculture Center Publication #7213. https://srac.tamu.edu/
DiMaggio, M. A., E. J. Cassiano, K. P. Barden, S. W. Ramee, C. L. Ohs, and C. A. Watson. 2017. "First Record of Captive Larval Culture and Metamorphosis of the Pacific Blue Tang, Paracanthurus hepatus." Journal of the World Aquaculture Society 48(3): 393–401.
Gopakumar, G., and I. Santhosh. 2009. "Use of Copepods as Live Feed for Larviculture of Damselfishes." Asian Fisheries Science 22(1): 1–6.
Groover, E. 2018. Assessment of Culture Techniques for Two Halichoeres Wrasses, H. melanurus and H. chrysus. Master's Thesis. University of Florida. Gainesville, FL, USA.
Hoff, F. H. 1996. Conditioning, Spawning, and Rearing of Fish with Emphasis on Marine Clownfish. Dade City, FL: Aquaculture Consultant Inc.
Hopkins, S., and C. S. Tamaru. 2005. "Manual for the Production of the Banggai Cardinalfish, Pterapogon kauderni, in Hawai'i." University of Hawaii School of Ocean and Earth Science and Technology.
Koldewey, H. J., and K. M. Martin-Smith. 2010. "A Global Review of Seahorse Aquaculture." Aquaculture 302(3): 131–152.
Lin, Q., J. Lin, and D. Zhang. 2008. "Breeding and Juvenile Culture of the Lined Seahorse, Hippocampus erectus Perry, 1810." Aquaculture 277(3): 287–292.
Madhu, K., R. Madhu, and T. Retheesh. 2016. "Spawning, Embryonic Development and Larval Culture of Redhead Dottyback Pseudochromis dilectus Lubbock, 1976 under Captivity." Aquaculture 459: 73–83.
Moe, M. A. 1982/1992. The Marine Aquarium Handbook: Beginner to Breeder. Plantation, FL: Green Turtle Publishing.
Moorhead, J. A., and C. Zeng. 2011. "Breeding of the Forktail Blenny Meiacanthus atrodorsalis: Broodstock Management and Larval Rearing." Aquaculture 318(1): 248–252.
Olivotto, I., A. Rollo, R. Sulpizio, M. Avella, L. Tosti, and O. Carnevali. 2006. "Breeding and Rearing the Sunrise Dottyback Pseudochromis flavivertex: the Importance of Live Prey Enrichment during Larval Development." Aquaculture 255(1): 480–487.
Olivotto, I., C. C. Piccinetti, M. A. Avella, C. M. Rubio, and O. Carnevali. 2010. "Feeding Strategies for Striped Blenny Meiacanthus grammistes Larvae." Aquaculture Research 41(9).
Rhyne, A. L., M. F. Tlusty, P. J. Schofield, L. Kaufman, J. A. Morris, and A. W. Bruckner. 2012. "Revealing the Appetite of the Marine Aquarium Fish Trade: The Volume and Biodiversity of Fish Imported into the United States." PLoS ONE 7(5): e35808.
Sazima, I., C. Sazima, R. B. Francini-Filho, and R. L Moura. 2000. "Daily Cleaning Activity and Diversity of Clients of the Barber Goby, Elacatinus figaro, on Rocky Reefs in Southeastern Brazil." Environmental Biology of Fishes 59(1): 69–77.
Smith-Vaniz, W. 2007. Saber-Toothed Blennies, Tribe Nemophini (Pisces: Blennidae). Academy of Natural Sciences.
Smith, W. L., and W. C. Wheeler. 2006. "Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms." Journal of Heredity 97(3): 206–217.
Thacker, C. E., A. R. Thompson, and D. M. Roje. 2011. "Phylogeny and Evolution of Indo-Pacific Shrimp-Associated Gobies (Gobiiformes: Gobiidae)." Molecular Phylogenetics and Evolution 59(1): 168–176.
Thresher, R. E. 1984. Reproduction in Reef Fishes. Neptune City, NJ: T.F.H. Publications.
Tlusty, M. 2002. "The Benefits and Risks of Aquacultural Production for the Aquarium Trade." Aquaculture 205: 203–219.
USDA (United States Department of Agriculture)-NASS (National Agricultural Statistics Service). 2019. Census of Agriculture 2017, Census of Aquaculture 2018. Volume 3, Special Studies, Part 2.
Vagelli, A. A. 2004. "Significant Increase in Survival of Captive‐bred Juvenile Banggai Cardinalfish Pterapogon kauderni with an Essential Fatty Acid‐Enriched Diet." Journal of the World Aquaculture Society 35(1): 61–69.
Victor, B. C. 1986. "Duration of the Planktonic Larval Stage of One Hundred Species of Pacific and Atlantic Wrasses (Family Labridae)." Marine Biology 90(3): 317–326.
Wittenrich, M. L., and P. L. Munday. 2005. "Bi-directional Sex Change in Coral Reef Fishes from the Family Pseudochromidae: An Experimental Evaluation." Zoological Science 22(7): 797–803.