Introduction
As the human population in Florida continues to grow, so does the demand for an expanded road network to accommodate daily needs for automobile travel.
Due to the many benefits that roads provide people, their construction has exploded in the last century. Although Florida only ranks twenty sixth for land area in the United States, it currently ranks seventh for its total road coverage, with approximately 275,376 miles (FHWA 2020). Road construction within the state is certain to increase in the future, given population growth.
This booming rate of road construction has led to increased concern about the ecological impacts caused by roads, which has resulted in its own field of research known as transportation or road ecology (Forman et al. 2003). Road ecology aims to quantify the impacts of roads on the natural environment and inform transportation planners about ways to avoid, minimize, or mitigate these impacts.
The purpose of this publication is to provide an overview of one specific aspect of road ecology: the ecological impacts of roads on Florida’s threatened and endangered species of vertebrates. First, we provide an overview of the types of impacts roads may have on imperiled wildlife. We then describe how threatened and endangered species in Florida are affected by roads and discuss potential ways to mitigate road impacts. We consider the impacts from all impervious roads, including one-lane and two-lane paved roads and highways, as well multi-lane interstates. Our focus is specific to federally listed species of amphibians, birds, mammals, and reptiles that occur in Florida (USFWS 2022; FWC 2021). The target audience of this publication is transportation engineers, planners, county Extension agents, land developers, and transportation agency employees.
Roads and Wildlife
The term “road effect zone” is commonly used to describe the area of impact extending out from the road into the surrounding landscape (Jackson 2000). The road effect zone includes the land area affected by both direct and indirect impacts. As a result, the road effect zone is disproportionately larger than the footprint of the road. For example, public roads are estimated to occupy one percent of the United States; however, their road effect zone is estimated to affect 20 percent of the landscape (Jackson 2000). Additionally, the extent of the road effect zone is positively correlated with road size, traffic volumes, and posted speed limits, with busy multi-lane interstates having the largest road effect zone and causing the greatest impacts (Ament et al. 2008; Jackson 2000).
The disproportionate impact that roads and their associated traffic have on the environment they occupy also extends to the local wildlife populations whose habitat is bisected by these roads (Jackson 2000). The negative impacts from roads are of particular concern for listed species whose populations are often small and already vulnerable because of other factors. The Endangered Species Act of 1973 (ESA) affords special protection to species that have been listed as either threatened or endangered. Endangered species are those on the verge of extinction, and threatened species are those on the verge of becoming endangered.
The ESA requires entities, both private and public, to evaluate all potential impacts to listed species before any major action that could cause them harm. It is therefore important for transportation engineers and state and federal transportation agencies to be aware of the major ecological impacts that roads can impose on amphibians, birds, mammals, and reptiles.
Impacts vary by species and include habitat loss, habitat fragmentation, road mortality, road avoidance, and disruption of movement patterns (Balkenhol and Waits 2009). Additionally, roads and their vehicular traffic can cause species to experience more stress even if they appear to habituate and remain in areas adjacent to roadways (Francis and Barber 2013). These impacts can interact with each other, resulting in aggregated cumulative impacts that can pose a threat to long-term survival over time.
Table 1. Potential impacts of roads on wildlife populations (adapted from Balkenhol and Waits 2009).
Habitat Loss
Habitat loss is the complete removal of an area available to be used as habitat and is relatively permanent. The construction of roads typically consists of concrete or asphalt lanes, and sodded roadside shoulders and/or medians. It often includes stormwater ditches or stormwater reservoirs. Additionally, the construction of roads has been linked to increasing urbanization, with roads attracting developers to previously unconnected and inaccessible areas (Demirel et al. 2008). On average, it is estimated that for every 0.62 mile of highway that is constructed, approximately 1591 acres of land will be developed or converted in some way (Ament et al. 2008), further exacerbating the amount of wildlife habitat lost (Figure 1). A smaller amount of habitat supports smaller wildlife populations because the number of available resources has decreased. This decreases the overall genetic diversity of the population, with fewer individuals contributing to the gene pool.
Additionally, future habitat loss is anticipated via the “coastal squeeze effect.” This occurs when man-made barriers, including roads, prevent the natural landward migration of wetlands and shorelines as sea levels rise. This “squeeze” effect has the same detrimental effects on wildlife populations as habitat loss described above.
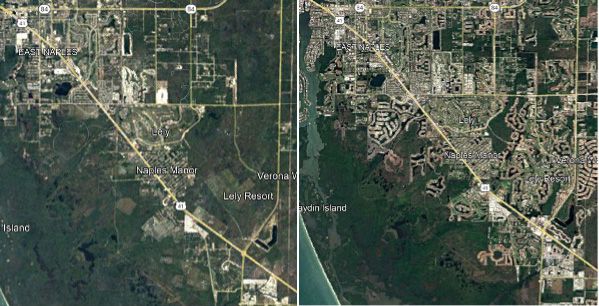
Credit: Google Earth Aerial Imagery
Fragmentation
Habitat fragmentation is when large patches of habitat are divided into smaller patches (Figure 2). Roads commonly bisect and fragment previously large tracts of wildlife habitat into smaller patches, increasing the amount of edge habitat. These edges are often continuously disturbed through the ongoing maintenance of roadside shoulders. Additionally, disturbed edges can provide opportunities for the establishment and spread of introduced exotic plant and animal species that can outcompete native wildlife (Dean et al. 2019; Jackson 2000).

Credit: S. A. Johnson, UF/IFAS
Fragmentation is not only a threat to habitats but also to wildlife populations. Roads can fragment wildlife populations into smaller isolated populations, reducing the overall population size and connectivity. For example, roads may artificially reduce the home-range size of individuals. This effect means that even if movement across the road is possible, individuals in the two fragmented populations may not interact or reproduce with each other (Taylor and Goldingay 2010). Moreover, smaller populations have a higher risk of local extinction, especially in the event of a natural or man-made disturbance (USFWS 2019d). For example, in a small population there is a higher chance that all individuals will be killed during a hurricane, becoming locally extinct.
Road Mortality
The most obvious impact that wildlife experience from roads is mortality from wildlife-vehicle collisions (Boston 2016). It is estimated that upwards of 365 million vertebrates are killed each year in the United States (FHWA 2008) from vehicle collisions. Road characteristics such as number of lanes, posted speed limits, traffic volumes, and the grade of the road (level or above the surrounding environment) influence the overall number and type of species that are vulnerable to wildlife-vehicle collisions (Dodd et al. 2004). Mammals, specifically small mammals (Figure 3), were found to be the most killed species along roads, followed by birds, then reptiles (Magioli et al. 2019; Gonzalez-Gallina et al. 2013). Wildlife-vehicle collisions were found to be the highest along paved two-lane roads (FHWA 2008). Collisions with large animals such as deer, bear, alligators, and panthers also pose a threat to human safety. Collisions with large animals can lead to serious damage or injury to vehicles and their drivers.
Direct mortality via roadkill may lead to a decrease in the local wildlife population size (Taylor and Goldingay 2010). This is particularly important for species that are long lived with low reproductive rates (FHWA 2008) and for threatened and endangered species. In these populations, the death of even a few individuals can reduce the breeding population (the number of individuals reproducing and contributing to the gene pool) and lead to local extinction (Jackson 2000; USFWS 1999; USFWS 2019d).

Credit: S. A. Johnson, UF/IFAS
Barrier Effect
Roads prevent many species from moving across the landscape as they naturally would (Figure 4). As a result, roads can isolate wildlife populations that are unable to cross the road (Ament et al. 2008). This aversion could be to the actual road itself or from the noises, fumes, or lights associated with roads and vehicular traffic (Fahrig and Rytwinski 2009). For example, the noises associated with roads act as a barrier to many bird species, and their populations tend to be reduced near roadways as a result (Grade and Sieving 2016). Species such as bats and birds use moonlight to navigate, however the artificial light sources along roadways could impair this night-time travel (Dean et al. 2019).

Credit: S. A. Johnson, UF/IFAS
Regardless of the reason for road avoidance, reduced movement across the landscape may result in the populations on either side of the roadway becoming genetically isolated. Genetic isolation can lead to inbreeding and reduced genetic diversity within the segmented populations. If there is less genetic diversity within a population, they will have less adaptive capacity and may not be able to survive new or changing conditions.
Habituation Effect
If species do not avoid the road but continue to inhabit adjacent habitat, they may appear to become habituated to the road and its associated vehicular traffic (Figure 5). Research suggests, however, that even species that continue to inhabit areas near roadways may still suffer ecological consequences. Chronic noise from vehicular traffic can interfere with a species ability to detect important sounds such as mating calls or the movement of a nearby predator (Francis and Barber 2013; Dean et al. 2019; Grade and Sieving 2016). Additionally, the presence of a frequent noise source causes many species to spend more of their energy being vigilant and less for foraging and reproduction (Francis and Barber 2013; Shannon et al. 2015). Less time foraging and mating further reduces fitness and reproductive success (Francis and Barber 2013).

Credit: S. A. Johnson, UF/IFAS
Florida’s Threatened and Endangered Species
Florida is home to 67 threatened and endangered animal species, with several that are endemic to (i.e., only found in) the habitats unique to Florida (USFWS 2022). The state of Florida also has one of the largest road networks with approximately 275,376 miles of roads, including several multilane highways that bisect portions of the state (FHWA 2020). Most roadways in Florida are approaching capacity, so new transportation corridors are being proposed. Impacts to threatened and endangered species are, therefore, a major concern.
The US Fish and Wildlife Service (USFWS) conducts reviews on threatened and endangered species and their population status every five years. Species were excluded from this publication if roads were not identified as a threat. These included interior species whose habitats were primarily protected, coastal species restricted to small habitats seaward of roads, seabirds, and completely marine or aquatic species (with the exception of sea turtles). Additionally, species thought to already be extinct were also excluded from this publication. Based on this information, roads were determined to be a major threat to a total of 29 federally threatened and endangered animal species in Florida, excluding fish and invertebrates (Table 2).
Table 2. Federally Threatened and Endangered Species Road Impact Matrix.
Amphibians
Frosted flatwoods salamander (T) and Reticulated flatwoods salamander (E)
The frosted flatwoods salamander (Ambystoma cingulatum) and the reticulated flatwoods salamander (Ambystoma bishopi) are small salamanders with very restricted ranges. The frosted flatwoods salamander occurs in north Florida east of the Apalachicola River in Franklin, Wakulla, Liberty, Jefferson, and Baker counties. The reticulated flatwoods salamander occurs in north Florida in all counties west of the Apalachicola River.
Both species have complex life cycles where the juveniles are primarily aquatic, and the adults are primarily terrestrial. Adults migrate to seasonally flooded wetlands (known as breeding ponds) to breed and lay their eggs each year. Females usually lay their eggs on or immediately below low-growing herbaceous vegetation and grasses.
In Escambia County, road construction destroyed an important breeding pond for the reticulated flatwoods salamander (USFWS 2020b). Roads have also fragmented and isolated previously large areas of habitat for both species and act as a major barrier to movement to and from their breeding ponds (USFWS 2020b). Both species also may be struck by vehicles while attempting to cross roads during migration to their breeding sites. Additionally, roads can alter the hydrology of the landscape, which could include potentially drying out breeding ponds, or, conversely, permanently inundating breeding ponds, which has implications for the quantity and type of vegetation present as well as the presence of aquatic predators.
Birds
Wood stork (T)
The wood stork (Mycteria americana) is a large bird that forages in wetlands and occurs throughout the state of Florida. Although wood stork numbers are increasing, road construction and widening is still a major threat to them because of the associated wetland habitat loss. Additionally, stormwater features associated with roads appear to attract wood storks. This has led to an increase in wood storks being involved in vehicle collisions (USFWS 2007). Moreover, while ditches and ponds may provide foraging habitat, they may be less favorable because of the potential stress vehicles and humans may cause.
Audubon’s crested caracara (T)
The Audubon’s crested caracara (Polyborus plancus audubonii), previously the northern crested caracara (Caracara cheriway), is a large raptor that inhabits prairies, pastures, and shrub and brushland in central and south Florida. Caracaras feed primarily on the carcasses of dead animals. They often forage along roads in search of recent roadkills and thus become victims of vehicle strikes, themselves. Vehicle collisions accounted for approximately 55% of mortalities to caracaras in a 1995 study (USFWS 2009), with juveniles more susceptible to being hit (USFWS 2009). Additionally, even though caracaras use roadways for foraging, they may still experience heightened stress and suboptimal foraging from the constant noise and threat of oncoming vehicles.
Florida scrub-jay (T)
The Florida scrub-jay (Aphelocoma coerulescens) is a highly territorial species that lives in cooperating family groups. This species is only found in oak scrub habitats throughout peninsular Florida. Oak scrub is becoming increasingly rare, and much of the remaining oak scrub habitat has already been fragmented by roads. Individuals have been observed crossing two-lane roads and establishing new territories (Mumme et al. 2000), suggesting smaller roads do not provide a barrier to movement.
In contrast, Florida scrub-jays may be attracted to roadsides because the lack of vegetation allows them to detect predators more easily and provides optimal foraging habitat (USFWS 2019d). However, these edges may also allow predators such as feral cats to locate scrub-jays and their nests more often (USFWS 2019d). Roadway noise was found to have no major negative or positive impact on Florida scrub-jays (Mumme et al. 2000). It is important to note the noise study only included smaller two-lane roads with lower traffic volumes. Larger roads and highways may provide some barrier to movement or alter nest success (USFWS 2019d).
The biggest roadway threat to the Florida scrub-jay is mortality via vehicle collisions. Mortality of individuals in territories adjacent to roads exceeded the number of individuals produced, meaning if immigration did not occur, the territory would go extinct (USFWS 2019d; Mumme et al. 2000). Individuals from interior territories that immigrate to roadside territories experience higher mortality, at least initially (Mumme et al. 2000). Additionally, young birds are commonly killed by passing vehicles because they have not yet learned to avoid them (Mumme et al. 2000).
Eastern black rail (T)
The eastern black rail (Laterallus jamaicensis ssp. jamaicensis) is a small marsh bird that inhabits coastal salt marshes in central and north Florida. While not immediately threatened by roads, rails may be limited by road-induced habitat loss. Wetlands, including saltwater marshes, can migrate across the landscape if there is no barrier to movement. With sea-level rise, salt marshes will naturally migrate landward; however, roads and other man-made barriers may prevent their establishment (USFWS 2019a). This effect is known as the “coastal squeeze” (USFWS 2019a). As a result, the amount of suitable habitat for the eastern black rail will be greatly reduced.
Mammals
Florida Key deer (T)
The Florida Key deer (Odocoileus virginianus clavium) is a small species of white-tailed deer restricted to the Florida Keys. Due to the amount of urbanization that has occurred in the Keys, there are few to no large areas of natural habitat left. To prevent further development in Monroe County, a building moratorium was enacted in 1995 (USFWS 2019c). As a result, future habitat loss is not very likely, and further habitat fragmentation is not a major threat.
However, roads still pose a major threat to Key deer through wildlife vehicle collisions. Key deer favor the open grassy areas along roads and are commonly seen foraging along roadsides (Roberts et al. 2010). The roads in the Florida Keys experience high levels of traffic year-round, increasing the chance of Key deer being killed by vehicles. In 1998, road mortality accounted for 67 percent of Key deer deaths (USFWS 1999). Additionally, even if Florida Key deer appear to be habituated to roads and traffic, they may experience reduced foraging and breeding behaviors than if in remote areas.
Key Largo cotton mouse (E), Key Largo woodrat (E), silver rice rat (E), and Lower Keys rabbit (E)
The Key Largo cotton mouse (Peromyscus gossypinus allapaticola), Key Largo woodrat (Neotoma floridana smalli), silver rice rat (Oryzomys palustris natator), and Lower Keys rabbit (Sylvilagus palustris hefneri) are discussed collectively because they are all small mammals restricted to the Florida Keys and face similar threats. The natural habitats on the Florida Keys have been fragmented into small, disconnected patches that may not support healthy populations of these small mammals (USFWS 1999).
Roads can act as a complete barrier to movement for these species. However, because their habitat patches are small, these species may have to disperse across roads to find food and mates, which increases their chances of being hit by vehicles (USFWS 1999). Additionally, the silver rice rat and the Lower Keys marsh rabbit inhabit wetlands that are threatened due to sea-level rise and may experience the “coastal squeeze” effect described above.
Beach Mice
There are five subspecies of beach mice that are listed in the state of Florida: the Perdido Key beach mouse (Peromyscus polionotus trissyllepsis), Anastasia Island beach mouse (P. p. phasma), St Andrew beach mouse (P. p. peninsularis), Choctawhatchee beach mouse (P. p. allophrys), and southeastern beach mouse (P. p. niveiventris). All are listed as endangered, except the southeastern beach mouse, which is listed as threatened. All are restricted to very small ranges along the coast of Florida and inhabit beach dune systems. Although roads are not a direct threat, they may limit the amount of future habitat that is available. Like wetlands, dunes can migrate and form farther inland (USFWS 2021b). However, roads will prevent this natural migration. Under sea-level rise, a significant amount of habitat might be lost because of the barrier that roads pose to dune reestablishment (USFWS 2021b). Additionally, because their populations are so small, these mice have a greater chance of becoming extinct from hurricanes that frequently impact Florida’s coastline (USFWS 2021b).
Florida panther (E)
The Florida panther (Puma concolor coryi) occurs in peninsular Florida from Orange County south to the Florida Keys. They can use many different types of habitats but prefer dense forests. Florida panthers have large home ranges and as a result travel long distances. The construction of roads has fragmented their habitat and introduced human access to these previously remote areas (USFWS 2020a).
Due to their large home ranges, panthers often need to cross roads. However, the long-term survival of the Florida panther is threatened by mortality from wildlife vehicle collisions (FHWA 2008). From 1982 through 2018, vehicle collisions accounted for 60% of panther mortalities (USFWS 2020a). Even in cases where a vehicle collision is not fatal, the injured panther may need to be removed from the population and placed in captivity, further reducing the population.
Florida bonneted bat (E) and gray bat (E)
The Florida bonneted bat (Eumops floridanus) is a large species of bat that occurs from south Florida north to Highlands County. One major threat to this species is loss of potential roosting habitat which includes pine trees and palm trees (USFWS 2009). Road construction involves the complete clearing of long linear corridors on the landscape and could destroy potential roost trees. The gray bat (Myotis grisescens) is a smaller bat restricted to the Florida Panhandle that roosts in caves and is not directly affected by habitat loss via road construction. However, both species of bats travel at night using echolocation to find prey and detect objects. Noise can interfere with bats’ ability to find and capture prey (Allen et al. 2021) and act as a barrier to movement across roads (Russo and Ancilloto 2015; Bennett and Zurcher 2013). Direct mortality from vehicle collisions is also a common threat (Russo and Ancilloto 2015), especially since flying bats are hard to see at night from a fast-moving vehicle.
Reptiles
Eastern indigo snake (T)
The eastern indigo snake is a large non-venomous snake that can be found in a variety of natural habitats throughout the state of Florida. The primary threats that roads pose to indigo snakes are habitat fragmentation and mortality from vehicles (USFWS 2019b). Indigo snakes will cross small, two-lane roads and are often killed while doing so, but they avoid larger multilane highways (USFWS 2019b; Bauder et al. 2018). This is a concern because indigo snakes have large home ranges and move long distances. It is estimated that >10,000 acres of unfragmented habitat is required to support a healthy population (USFWS 2019b).
Roads have increasingly fragmented eastern indigo snake habitats into smaller, unsuitable patches, requiring the snakes to traverse roads and increasing their chances of becoming roadkill. Additionally, when the weather is cool, eastern indigo snakes are actually attracted to roads. Snakes bask to regulate their body temperature, and roads, being flat, unshaded, and relatively dark-colored, soak up the sun and radiate heat, making them ideal basking spots (Enge and Wood 2002). At one study site in Florida, the eastern indigo snake declined approximately 95% between 1983 and 2009 (Godley and Moler 2013). Road mortality was suspected to be a major factor in their decline (Godley and Moler 2013).
Atlantic salt marsh snake (T)
The range of the Atlantic salt marsh snake (Nerodia clarkii taeniata) is highly limited to the coastal saltwater marshes in Volusia and southern Flagler Counties. As in the case of the eastern black rail discussed above, sea-level rise may limit the amount of future habitat that is available through the “coastal squeeze” effect where roads act as a barrier to marsh migration (USFWS 2019). As a result, the amount of suitable habitat for the Atlantic salt marsh snake will likely be greatly reduced.
American crocodile (T) and American alligator (T(S/A))
The American crocodile (Crocodylus acutus) inhabits saltwater and brackish areas in southern Florida. The American alligator (Alligator mississippiensis) is listed as threatened due to its similarity of appearance with the American crocodile. Crocodilians are “cold-blooded” reptiles that derive warmth from their environment and will bask on roadsides to seek warmth from the sun. Although roads fragment their habitats, they are often seen crossing roads or using culverts to travel between fragmented areas. Because neither species is known to avoid roads, they are susceptible to being hit by vehicles (USFWS 1999). American crocodiles are sensitive to disturbance from humans (USFWS 1999), suggesting that vehicular noise may cause stress if they inhabit areas adjacent to roadways.
Blue-tailed mole skink (T) and sand skink (T)
The blue-tailed mole skink (Plestiodon egregius lividus) and the sand skink (Plestiodon reynoldsi) are small lizards that are restricted to the high, dry, sandy ridges of central Florida. They require very dry soils in which they burrow for shelter and forage for small invertebrates. Because this high and dry habitat is prime real estate for developers, much of their habitat has been lost and fragmented by urbanization (USFWS 2021a). Skinks are small, burrowing animals, and roads act as a complete barrier to their movement, hemming in and isolating their populations. These effects have the potential to adversely impact their genetic diversity (USFWS 2021a), which decreases a species’ ability to adapt to changing environmental conditions and could lead to reduced chances of long-term survival.
Sea turtles
There are three species of sea turtles that have significant nesting populations on Florida’s coastline: the loggerhead sea turtle (Caretta caretta), green sea turtle (Chelonia mydas), and leatherback sea turtle (Dermochelys coriacea). Loggerhead and green sea turtles are listed as threatened and nest along the east and west coasts of Florida. The leatherback sea turtle is listed as endangered and primarily nests along the east coast of Florida. Although sea turtles are primarily marine animals, females come ashore at night to lay their eggs on Florida’s beaches.
Roadways can degrade sea turtle nesting habitat by increasing noise and light pollution (NMFS/USFWS 2007). Sea turtles use visual cues to navigate to and from the nest site. Road lighting can impede navigation cues and disorient both nesting females and just-hatched baby turtles. Female turtles may be deterred from leaving the sea to make nests and lay eggs if the beach is too bright (Witherington 1992). When they emerge from the nest, sea turtle hatchlings instinctively search for bright light to help them find the sea. Historically, of course, the brightest light in the sky was always the moon, and the moon always reliably led the little turtles to the sea.. But on many beaches today, street lights are brighter than the moon, so it is common for baby turtles to turn their backs on the ocean and crawl towards bright streetlights, and, inevitably, onto roads, where they may be crushed by passing vehicles (McFarlane 1963; Broadwell et al. 2001). Even if they are not killed on the road, lost baby turtles are unlikely to be able find the ocean, and many die from dehydration, exhaustion, or predation.
Sea turtle nesting habitat is also threatened by the coastal squeeze effect. Roads prevent the beach and dune systems from naturally migrating inland as sea level rises. This effect will significantly reduce the amount of nesting habitat for sea turtles in Florida. The majority of loggerhead and virtually all green turtle and leatherback nesting in the United States occurs in Florida. Losing Florida’s beaches as nesting habitat from the coastal squeeze effect will significantly reduce sea turtle populations, not only in Florida but all over the United States.
Potential Mitigation Options
Given the wide-ranging impacts that roads and vehicular traffic cause, engineering effective mitigation strategies to alleviate these impacts can help conserve Florida’s threatened and endangered species. Each potential impact may require a different strategy depending on the habitat type, species, road density, and size of the road(s). As a result, a suite of mitigation strategies is required to be effective.
Mitigation Strategies
- Improve existing roads or use existing linear corridors for new roads.
- Protect adjacent habitat to prevent further habitat loss.
- Plan for ecological connectivity across the landscape.
- Install wildlife fencing and wildlife crossing structures.
- Reduce speed limits.
- Install wildlife crossing signs.
- Provide educational or outreach material to landowners or homeowners.
- Improve road and vehicle technology to reduce noise.
- Turn off lights, use shields, and install lights with longer wavelengths.
- Finance wildlife research studies to learn more about roadway impacts.
Planning to improve road configuration across the landscape is important to reduce and mitigate habitat loss and fragmentation impacts. Wildlife population persistence was found to be higher when all traffic and roads are bundled close together instead of spreading out traffic and roads across the landscape (Figure 6, Jaeger et al. 2005). This approach preserves larger tracts of undisturbed habitats that can support larger wildlife populations (Jaeger et al. 2005). Therefore, new road corridors should be avoided to the greatest extent possible. Instead, widening existing roads to increase traffic capacity is preferable. If a new corridor is unavoidable, using areas near existing linear corridors (including roadways, railways, and canals) is preferred (Jaeger et al. 2005).
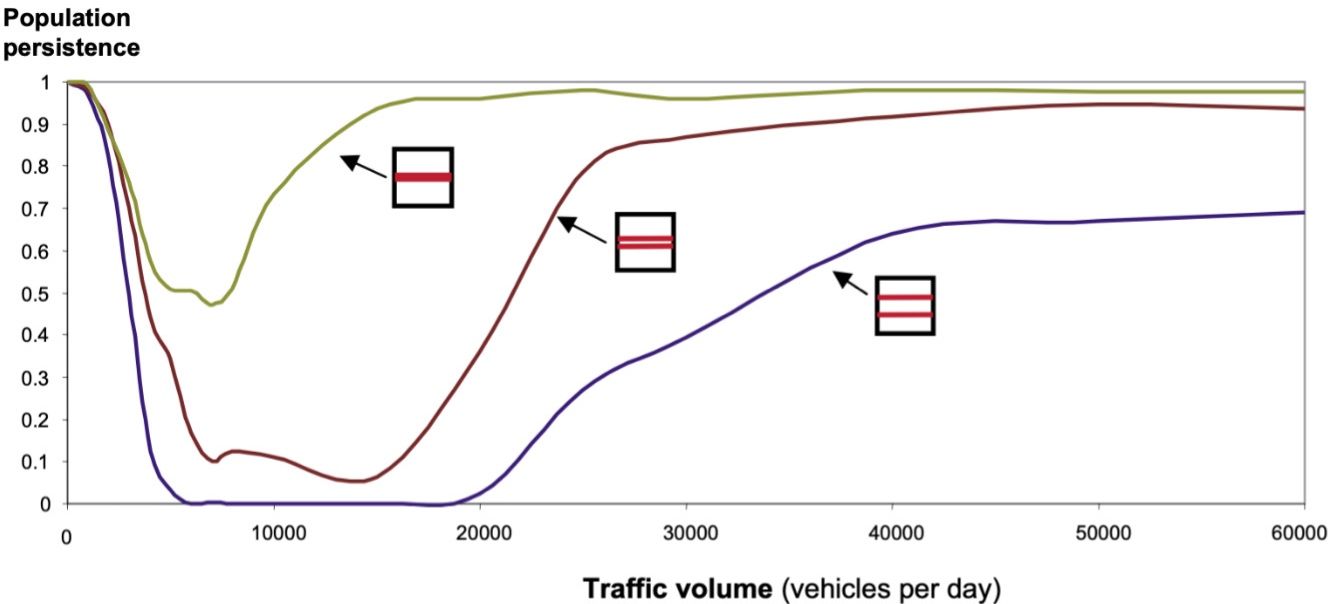
Credit: Figure 9 from Jeager et al. 2005
Another way to mitigate for habitat loss and fragmentation is to protect adjacent undeveloped habitat. Protecting habitat next to roads will reduce the urbanization of undeveloped areas to which roads provide new access. It can be done by purchasing land and putting it into a conservation easement or by donating undeveloped land to a local land management agency or conservancy.
Both wildlife vehicle collisions and the barrier effect contribute to the reduction, isolation, and subdivision of populations (Jaeger et al. 2005). There are two primary methods to mitigate these impacts: 1) modifying human behavior or 2) directing wildlife movement (FHWA 2008). Human behavior modification strategies include reducing speed limits, installing signage to inform drivers of the presence of wildlife (FHWA 2008) and outreach strategies. However, signs and speed limits alone are not effective (Seburn and McCurdy-Adams 2019).
Wildlife movement modification strategies include wildlife fencing and crossing structures (FHWA 2008). Wildlife fencing prevents animals from crossing the roadway and can greatly reduce the number of wildlife vehicle collisions (FHWA 2008). However, fencing alone further exacerbates the barrier effect of roads (Jaeger et al. 2005). Fencing that funnels wildlife towards wildlife crossing structures can reduce the number of wildlife vehicle collisions while allowing for movement across the landscape (FHWA 2008; Dodd et al. 2004; Jaeger et al. 2005). For example, fencing and wildlife crossing structures were installed for the Florida Key deer during the widening of US Highway 1 in the Florida Keys, successfully mitigating the increased wildlife vehicle collision threat from the additional lane (USFWS 2019c).
Wildlife crossing structures include overpasses (wildlife bridges) and underpasses (bridges, culverts, pipes, and tunnels). These structures, though effective when used with fencing or barrier walls, are typically the most expensive mitigation option (Jackson and Griffin 2000; Jaeger et al. 2005). Initial costs may be high, but wildlife crossing structures provide substantial cost savings over time. Savings increase each year that vehicle collisions with wildlife are reduced (Lister et al. 2015). Moreover, wildlife crossing structures benefit not just wildlife but people, as well. As anyone knows who has hit a deer, been startled into a skid when a raccoon leapt into the road, or smashed into an enormous, slumbering reptile while driving 50+ mph at night, keeping animals off the roads makes the roads much safer (Lister et al. 2015).
Additionally, the costs of wildlife crossing structures can be reduced. Existing features along the road corridor can be retrofitted for wildlife crossing. For example, extending bridges to ensure there is sufficient land area along rivers or wetlands can allow terrestrial wildlife to cross underneath bridges (FHWA 2008). Culverts or drainage pipes can be retrofitted with shelves to allow small animals to cross underneath the roadway.

Credit: Google, 2022 U.S. Geological Survey Map Data
The Florida Fish and Wildlife Conservation Commission recommends that lights adjacent to nesting beaches have longer wavelengths, be low to the ground, and be shielded from view on the beach to reduce the disorienting effect of streetlights on nesting sea turtles and their hatchlings. Lights embedded into the road kept the adjacent beach dark. In fact, they were found to be just as effective for turtle preservation as no lights, at all (Bertolotti and Salmon 2005).
Mitigating for the habituation effect that roads and traffic may cause is more problematic. Noise, artificial lights, and increased proximity to people and infrastructure can all harm wildlife. Reducing the number or intensity of lights can reduce overall light pollution and minimize adverse impacts. Moreover, improving road and vehicle technology to reduce traffic noise may minimize the number of perceived threats.
Cost-effective mitigation strategies can be developed only if engineers, transportation planners, ecologists, and biologists consult with one another and coordinate their efforts. Local biologists and ecologists can identify key habitat or breeding areas that should be avoided and provide information on the species that may be hurt. Certain species may require specific design features in crossing structures such as placement, size, noise, temperature, light, and bottom substrate for mitigation to be successful (Jackson and Griffin 2000). For example, larger mammals prefer open passageways with little or no vegetation, whereas small mammals and reptiles prefer vegetation cover (Taylor and Goldingay 2010).
Lastly, it is important to know how effective these mitigation strategies are. Does reducing the speed limit decrease wildlife vehicle collisions? Do fencing and crossing structure eliminate the barrier to movement? Do darker, quieter roads reduce stress in animals? Answers to these questions are particularly important for Florida’s threatened and endangered species. Unfortunately, not much is known on how much these strategies alleviate impacts to listed species. More research is greatly needed to determine the effectiveness of these actions. Transportation agencies should finance long-term research and monitoring that is targeted at answering these questions and, ultimately, alleviating these agencies’ regulatory burden.
Overall, the goal is to protect, conserve, and ultimately recover Florida’s threatened and endangered species. Recovering listed species will reduce the regulatory requirements for future roadway and transportation projects. However, simply complying with the minimum requirements of the ESA may not recover listed species back to healthy populations. Eliminating excess mortality and increasing ecological connectivity is critical to ensuring these populations increase and become self-sustaining. In this regard, transportation planners and engineers can play a key role in conserving and recovering these species through proactive regional planning, long-term research, and innovative mitigation strategies.
References
Allen, L. C., N. I. Hristov, J. J. Rubin, J. T. Lightsey, and J. R. Barber. 2021. Noise Distracts Foraging Bats. Proceedings of the Royal Society B 288: 20202689. https://doi.org/10.1098/rspb.2020.2689
Ament, R., A. P. Clevenger, A. Yu, and A. Hardy. 2008. “An Assessment of Road Impacts on Wildlife Populations in U.S. National Parks.” Environmental Management. 42:480–496. https://doi.org/10.1007/s00267-008-9112-8
Balkenhol, N., and L. P. Waits. 2009. “Molecular Road Ecology: Exploring the Potential of Genetics for Investigating Transportation Impacts on Wildlife.” Molecular Ecology. 18:4151–4164. https://doi.org/10.1111/j.1365-294X.2009.04322.x
Bauder, J. M. 2019. “Population Viability and Connectivity of the Federally Threatened Eastern Indigo Snake in Central Peninsular Florida.” Doctoral Dissertations. University of Massachusetts Amherst. https://doi.org/10.7275/xnh7-9s97
Bennett, V. J., and A. A. Zurcher. 2013. “When Corridors Collide: Road-Related Disturbance in Commuting Bats.” The Journal of Wildlife Management 77:93–101. https://doi.org/10.1002/jwmg.467
Bertolotti, L., and M. Salmon. 2005. “Do embedded roadway lights protect sea turtles?” Environmental Management 36 (5): 702–710. https://doi.org/10.1007/s00267-004-0288-2
Boston, K. 2016. “The Potential Effects of Forest Roads on the Environment and Mitigating Their Impacts.” Current Forestry Reports 2:215–222. https://doi.org/10.1007/s40725-016-0044-x
Broadwell, A., M. Salmon, and R. Ellis. 2001. “Dark Beaches – FDOT’s Approach to Resolving Coastal Roadway Lighting and Its Impacts to Adjacent Sea Turtle Nesting Beaches.” ICOET 2001 Proceedings.
Dean, R. J., C. L. Seymour, G. S. Joseph, and S. H. Foord. 2019. “A Review of the Impacts of Roads on Wildlife in Semi-Arid Regions.” Diversity 11 (5): 81. https://doi.org/10.3390/d11050081
Demirel, H., E. Sertel, S. Kaya, and D. Z. Seker. 2008. “Exploring Impacts of Road Transportation on Environment: A Spatial Approach.” Desalination. 226:279–288. https://doi.org/10.1016/j.desal.2007.02.111
Dodd, K. C. Jr., W. J. Barichivich, and L. L. Smith. 2004. “Effectiveness of a Barrier Wall and Culverts in Reducing Wildlife Mortality on a Heavily Traveled Highway in Florida.” Biological Conservation. 118:619–631. https://doi.org/10.1016/j.biocon.2003.10.011
Enge, K. M., and K. N. Wood. 2002. “A Pedestrian Road Survey of an Upland Snake Community in Florida.” Southeastern Naturalist 1 (4): 365–380. https://doi.org/10.1656/1528-7092(2002)001[0365:APRSOA]2.0.CO;2
Forman, R. T. T, D. Sperling, J. A. Bissonette, A. P. Clevenger, C. D. Cutshall, V. H. Dale, L. Fahrig, et al. 2003. Road Ecology: Science and Solutions. Island Press, Washington D.C.
Fahrig, L., and T. Rytwinski. 2009. “Effects of Roads on Animal Abundance: An Empirical Review and Synthesis.” Ecology and Society 14 (1): 21. https://doi.org/10.5751/ES-02815-140121
Federal Highway Administration (FHWA). 2008. Wildlife Vehicle Collision Reduction Study. Report FHWA-HRT-08-034. U.S. Department of Transportation.
FHWA. 2020. Highway Statistics 2019 website. Accessed March 6, 2020 https://www.fhwa.dot.gov/policyinformation/statistics/2019/hm60.cfm
Florida Fish and Wildlife Conservation Commission (FWC). 2021. Florida’s Official Endangered and Threatened Species List. https://myfwc.com/media/1945/threatened-endangered-species.pdf
Francis, C. D., and J. R. Barber. 2013. “A Framework for Understanding Noise Impacts on Wildlife: An Urgent Conservation Priority.” Frontiers in Ecology and the Environment 11 (6): 305–313. https://doi.org/10.1890/120183
Grade, A. M., and K. E. Sieving. 2016. “When the Birds Go Unheard: Highway Noise Disrupts Information Transfer between Bird Species.” Biology Letters. 12:20160113 https://doi.org/10.1098/rsbl.2016.0113
Godley, J. S., and P. E. Moler. 2013. “Population Declines of Eastern Indigo Snakes (Drymarchon couperi) over Three Decades in the Gulf Hammock Wildlife Management Area, Florida, USA.” Herpetological Conservation and Biology 8:359–365.
González-Gallina, A., G. Benítez-Badillo, O. R. Rojas-Soto, and M. G. Hidalgo-Mihart. 2013. “The Small, the Forgotten and the Dead: Highway Impact on Vertebrates and its Implications for Mitigation Strategies.” Biodiversity and Conservation 22 (2): 325–342. https://doi.org/10.1007/s10531-012-0396-x
Jackson, S. D. 2000. “Overview of Transportation Impacts on Wildlife Movement and Populations.” In Wildlife and Highways: Seeking Solutions to an Ecological and Socio-economic Dilemma, edited by T. A. Messmer and B. West, 7–20. The Wildlife Society.
Jackson, S. D., and C. R. Griffin. 2000. “A Strategy for Mitigating Highway Impacts on Wildlife.” Wildlife and Highways: Seeking Solutions to an Ecological and Socio-economic Dilemma, edited by T. A. Messmer and B. West, 143–159. The Wildlife Society.
Jaeger, J. A. G., L. Fahrig, and K. C. Ewald. 2005. “Does the configuration of road networks influence the degree to which roads affect wildlife populations?” ICOET 2005 Proceedings.
Lister, N. M., M. Brocki, and R. Ament. 2015. “Integrated Adaptive Design for Wildlife Movement under Climate Change.” Frontiers in Ecology and the Environment. 13 (9): 493–502. https://doi.org/10.1890/150080
Magioli, M., A. A. Bovo, M. P. Huijser, F. D. Abra, R. A. Miotto, V. H. Andrade, A. M. Nascimento, M. Z. Martins, and K. M. Micchi de Barros Ferraz. 2019. “Short and Narrow Roads Cause Substantial Impacts on Wildlife.” Oecologia Australis 23 (1): 99–111. https://doi.org/10.4257/oeco.2019.2301.09
McFarlane, R. W. 1963. “Disorientation of Loggerhead Hatchlings by Artificial Road Lighting.” Copeia 1963 (1): 153. https://doi.org/10.2307/1441283
Mumme, R. L., S. J. Schoech, G. E. Woolfenden, and J. W. Ftizpatrick. 2000. “Life and Death in the Fast Lane: Demographic Consequences of Road Mortality in the Florida Scrub-Jay.” Conservation Biology 14 (2): 501–512. https://doi.org/10.1046/j.1523-1739.2000.98370.x
National Marine Fisheries Service and U.S. Fish and Wildlife Service. 2007. 5-Year Review of Loggerhead Sea Turtle.
Roberts, C. W., B. L. Pierce, A. W. Braden., R. R. Lopez, N. J. Silvy, P. A. Frank., and D. Ransom Jr. 2010. “Comparison of Camera and Road Survey Estimates for White-Tailed Deer.” The Journal of Wildlife Management 70 (1): 263–267. https://doi.org/10.2193/0022-541X(2006)70[263:COCARS]2.0.CO;2
Russo, D., and L. Ancillotto. 2015. “Sensitivity of Bats to Urbanization: A Review.” Mammalian Biology 80: 205–212. https://doi.org/10.1016/j.mambio.2014.10.003
Schwab, A. C., and P. A. Zandbergen. 2011. “Vehicle-Related Mortality and Road Crossing Behavior of the Florida Panther.” Applied Geography 31: 859–970. https://doi.org/10.1016/j.apgeog.2010.10.015
Seburn, D. C., and H. McCurdy-Adams. 2019. “Do turtle warning signs reduce roadkill?” Canadian Field-Naturalist 133 (3): 216–220. https://doi.org/10.22621/cfn.v133i3.2279
Shannon, G., M. F. McKenna, L. M. Angeloni, K. R. Crooks, K. M. Fristrup, E. Brown, K. A. Warner, et al. 2015. “A Synthesis of Two Decades of Research Documenting the Effect of Noise on Wildlife.” Biological Reviews 91:982–1005 https://doi.org/10.1111/brv.12207
Taylor, B. D., and R. L. Goldingay. 2010. “Roads and Wildlife: Impacts, Mitigation and Implication for Wildlife Management in Australia.” Wildlife Research 37:320–331. https://doi.org/10.1071/WR09171
U.S. Fish and Wildlife Service (USFWS). 1999. Multi-Species Recovery Plan for South Florida.
USFWS. 2007. Wood Stork (Mycteria americana) 5-Year Review. Southeast Region. Jacksonville Ecological Services Field Office. Jacksonville, Florida.
USFWS. 2009. 5-Year Review of the Northern Crested Caracara. Southeast Region. South Florida Ecological Services Field Office. Vero Beach, Florida.
USFWS. 2019a. Species Status Assessment Report for the Eastern Black Rail (Laterallus jamaicensis jamaicensis), Version 1.3. August 2019. Atlanta, GA.
USFWS. 2019b. Species Status Assessment Report for the Eastern Indigo Snake (Drymarchon couperi). Version 1.1, July 2019. Atlanta, Georgia.
USFWS. 2019c. Species Status Assessment Report for the Florida Key Deer (Odocoileus virginianus clavium). Version 3.2, February 2021. Atlanta, Georgia.
USFWS. 2019d. Species Status Assessment Report for the Florida Scrub-Jay (Aphelocoma coerulescens). North Florida Ecological Services Office. Jacksonville, Florida.
USFWS. 2020a. Species Status Assessment for the Florida Panther. Version 1.0. September 2020. Vero Beach, Florida.
USFWS. 2020b. Species Status Assessment for the Frosted Flatwoods Salamander (Ambystoma cingulatum). Version 1.0. March 2020. Panama City Field Office.
USFWS. 2021a. Blue-tailed Mole Skink (Eumeces egregious lividus) 5-Year Review. July 2021. South Atlantic-Gulf Region. Florida Ecological Services Field Office. Vero Beach, Florida.
USFWS. 2021b. 5-Year Review of the Perdido Key Beach Mouse (Peromyscus polionotus trissyllepsis). Southeast Region. Panama City Field Office. Panama City, Florida.
USFWS. 2022. Listed species believed to or known to occur in Florida. Environmental Conservation Online System, Species Listings by State. https://ecos.fws.gov/ecp/report/species-listings-by-state?stateAbbrev=FL&stateName=Florida&statusCategory=Listed
Witherington, B. E. 1992. “Behavioral Responses of Nesting Sea Turtles to Artificial Lighting.” Herpetological 48 (1): 31–39.