Ticks: A small but mighty problem
Ticks are vectors of several diseases that affect humans. In the United States, the Center for Disease Control and Prevention (CDC) has reported an increasing number of tick-borne illnesses in humans (Figure 1; Wormser et al. 2020). Tick geographic ranges in the United States are expanding past historical boundaries due to environmental change (Esteve‐Gassent et al. 2016; Sonenshine 2018). In the southeastern United States, there are five common disease-transmitting tick species (Figure 2). The American dog tick, blacklegged tick, lone star tick, and brown dog tick are widely distributed in the eastern United States (although the brown dog tick has a worldwide distribution). The Gulf Coast tick is found in the southeastern and southern midwestern United States, although its range is expanding. These species are vectors of diseases such as ehrlichiosis, Lyme disease, spotted fever, and Rocky Mountain fever (Mayo Clinic 2022). Although there are many more tick species worldwide, this publication focuses on these five commonly encountered species (Leonovich 2015; Burtis et al. 2019).
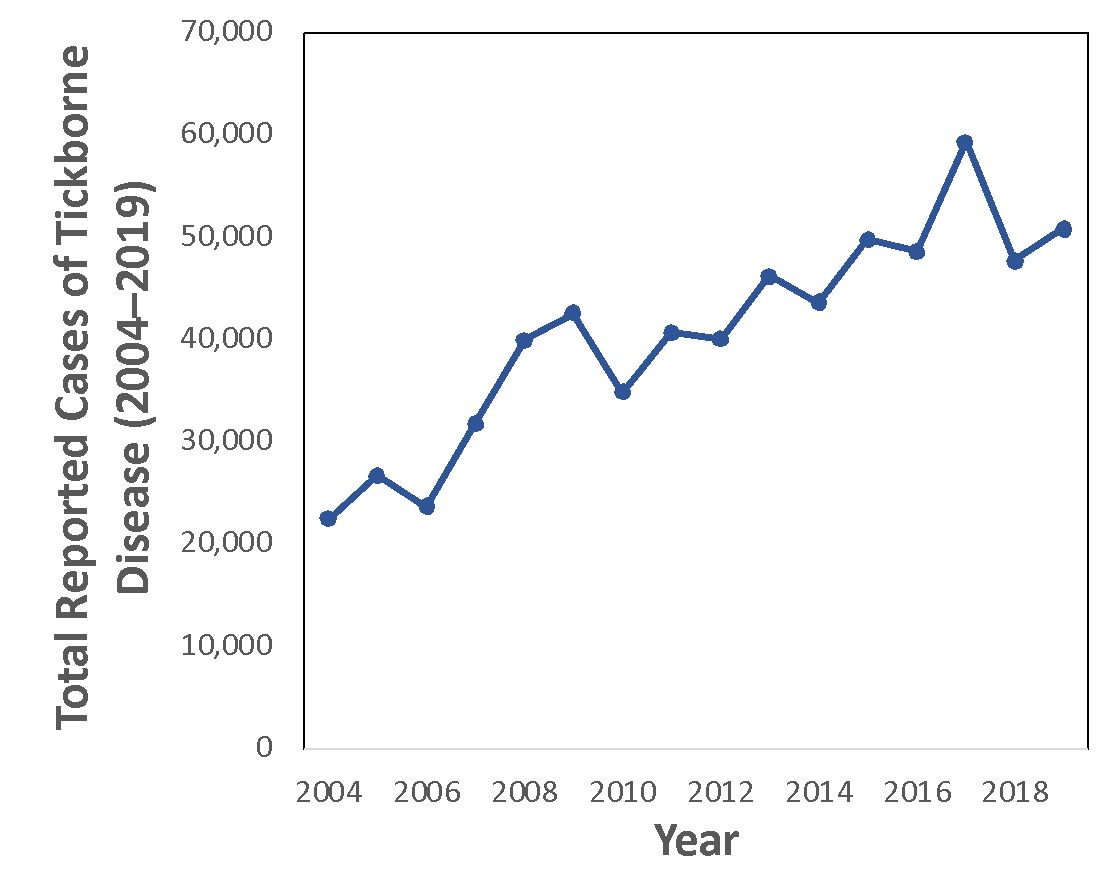
Credit: Data from CDC,https://www.cdc.gov/ticks/data-summary/index.html
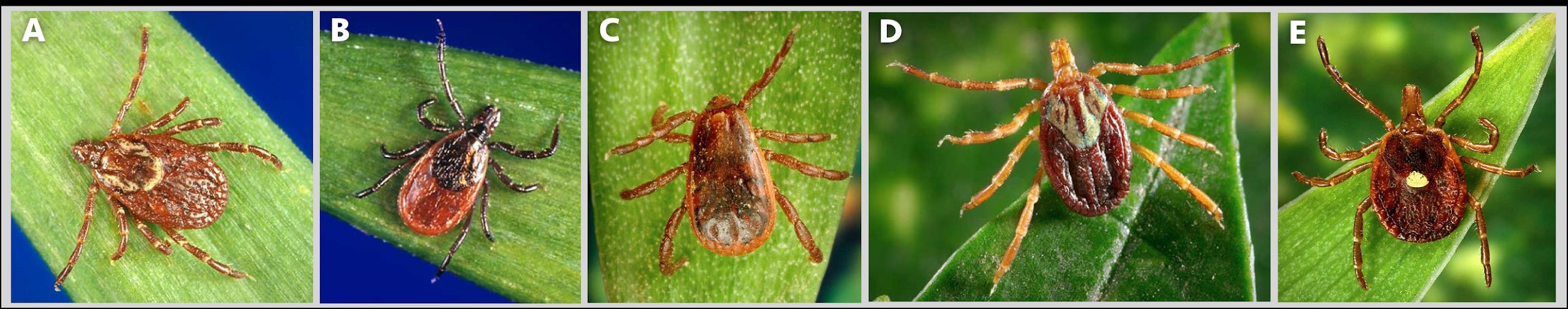
Credit: Jim Gathany, CDC, https://www.cdc.gov/ticks/geographic_distribution.html
To prevent the spread of disease, land managers and health officials seek affordable and environmentally friendly ways to control ticks (Mader et al. 2021). Prescribed fire is already an effective tool for managing vegetation and wildlife. Prescribed fires can kill ticks or decrease their populations when the habitat changes after repeated fires (Allen 2009; Gleim et al. 2019; Gallagher et al. 2022). By showing how fire can affect tick populations, this publication should help fire and natural resource managers, landowners, and interested individuals learn more about the usefulness of prescribed fires for controlling tick populations in the southeastern United States.
Tick Life Cycle
Ticks pass through several stages during their life cycle: egg, larva, nymph, and adult. Each stage can last anywhere from a few months to several years (Figure 3). To transition to the next stage, all five tick species must attach to a host and feed on its blood for at least a few days before dropping off. Four of the five species require a different host for each life stage. The exception is the brown dog tick, which can feed on the same animal throughout its life cycle. All stages of the brown dog tick prefer dogs as a host species, but nymphs and larvae of the other four tick species tend to feed on birds and small mammals and are sometimes found on amphibians and reptiles (Barnard 1986; Gleim 2020). Adult ticks feed on larger mammals such as horses, humans, dogs, and coyotes; adult blacklegged and lone star ticks primarily attach to white-tailed deer but can feed on other large mammals.
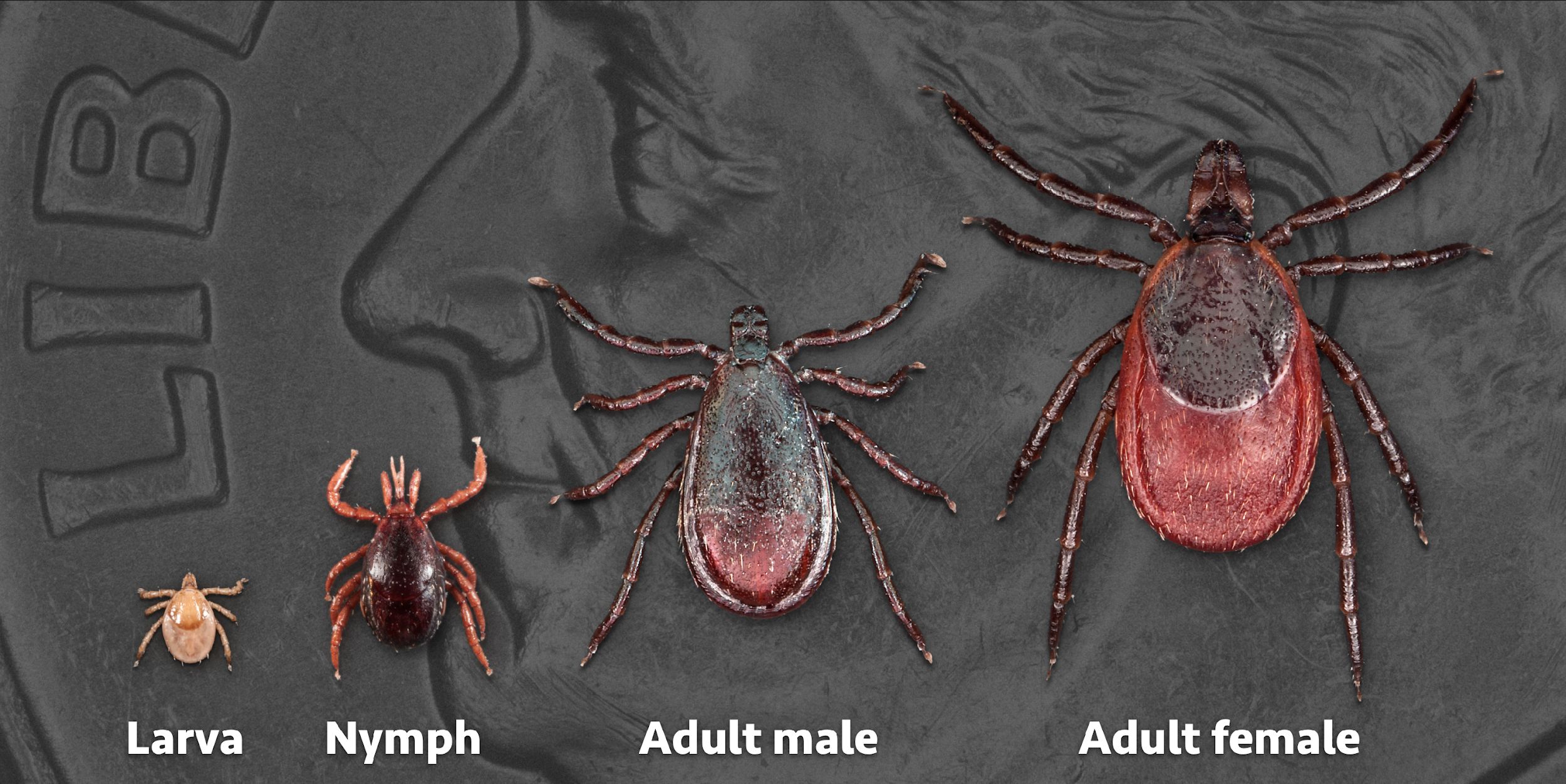
Credit: CDC
Ticks acquire and transmit pathogens while feeding on their host (CDC 2020). Their propensity to feed on numerous hosts increases the likelihood that they will acquire and transmit diseases between individual hosts, including humans. Hosts that serve as reservoirs for pathogens include mice species in the genus Peromyscus, the eastern chipmunk, masked shrew, short-tailed shrew (Gallagher et al. 2022), and dogs (Mather and Coughlin 1994). Although they are not considered to carry pathogens, some hosts, such as white-tailed deer and eastern turkey, can amplify tick species that carry diseases (Gallagher et al. 2022).
Habitat conditions are important for tick survival and for encountering hosts. Larvae, nymphs, and adults of all five species display “questing” or host-seeking behavior, which often involves climbing vegetation and waiting for hosts to pass (Figure 4; Arsnoe et al. 2015). Ticks can sense their host by smell, body heat, and vibrations or air movement. All five species are most active during the warmer months, with the specific time of peak activity depending on location (e.g., inland or coastal, northern or southern) (Teel et al. 2010; Leonovich 2015; Burtis et al. 2019). American dog ticks and blacklegged ticks overwinter in the soil (Burg 2001) or on the ground under litter, but these species and the others might remain active if temperatures are warm enough. Not all ticks or stages require the same environmental conditions. For example, humid conditions, such as dense, moist litter, are generally favorable for eggs of American dog ticks and blacklegged ticks. Gulf Coast tick larvae, nymphs, and adults prefer dry environments such as prairies and coastal uplands, while brown dog ticks can be found under a variety of conditions, including those of woodlands, houses, and dog kennels.

Credit: David Godwin, UF/IFAS
Ticks and Fire
Fire affects ticks by directly killing them and by changing their habitat and thus limiting their ability to find shelter or encounter hosts (Gallagher et al. 2022). For example, heat from a fire kills ticks, but environmental changes after fire (e.g., less vegetation or litter) can also decrease tick survival and questing success. Changes to habitat from fire can also change the behavior of tick hosts and predators in ways that affect ticks.
Direct Effects of Fire on Tick Populations
The heat from prescribed fires can be hot enough or last long enough to kill ticks. Temperatures >200°C for longer than 2.5 seconds can kill all tick life stages (Barnard 1986). Evidence shows that ticks may seek refuge in the leaf litter of unburned patches and other moist places to escape the heat produced by fires (Schulze et al. 1995). Thus, several studies have shown that a single prescribed burn is insufficient to reduce tick populations significantly (Barnard 1986), but long-term repeated burning maintains low tick numbers (Gleim et al. 2014).
Indirect Effects of Fire on Tick Habitat
Fire also changes tick habitat. By burning off or reducing vegetation, especially the humid litter layer, fire can promote hotter and drier conditions, decreasing available shelter and questing habitat (Callaham et al. 2003; Rosendale et al. 2017). Such conditions can negatively affect eggs, nymphs, and larvae of ticks that need moist conditions to survive (Schulze et al. 1995). For example, removing leaf litter reduces the abundances of all stages of lone star ticks; in one study, clearing vegetation reduced the abundance of larval lone star ticks by 72% two years after the fire (Hair and Howell 1970). However, because American dog ticks and Gulf Coast ticks are more tolerant of dry conditions, they might be less affected by vegetation removal (Gleim et al. 2013; White and Gaff 2018). Additionally, because brown dog ticks can also live inside homes and other structures, they might be less directly affected by changes to outdoor environments.
Effects of Fire on Tick–Host Encounters
Tick hosts use recently burned areas in different ways. For instance, white-tailed deer, a common host of blacklegged and lone star ticks, will avoid recently burned areas but gradually increase use as fresh forage returns (Fill and Crandall 2018). This could result in fewer encounters shortly after fire, but more host encounters and increasing tick abundance over time (Ivey and Clausey 1984; Allen 2009; Willis et al. 2012). Experimentally eliminating deer has been shown to decrease populations of blacklegged tick larvae and nymphs (Wilson et al. 1988). Wild turkeys also tend to forage in recently burned areas (Fill & Crandall 2018). Turkey poults foraging in recently burned areas were found to have fewer lone star ticks than those in unburned areas (Jacobson and Hurst 1979). However, small mammals, such as rodents, tend to use recently burned areas less, possibly because of greater chances of predation (Greenberg et al. 2007). Other mammals, such as racoons, may avoid recently burned areas because there is less food (Jones et al. 2004; Jorge et al. 2020). Thus, shifts in host availability over time can influence tick population size (Barnard 1986).
Effects of Fire on Tick Predators
Fires can increase populations of tick predators such as northern bobwhite quail, turkey, and arthropods (Samish and Alexeev 2001, Ginsberg et al. 2021). More predators may temporarily reduce tick populations and associated illnesses (Patterson and Knapp 2018). For example, population booms of northern bobwhite quail, which benefit from fire, have been correlated with decreases in Lyme disease cases the following year. Alternatively, fire can reduce predator abundance by killing predators or changing their habitat (Callaham et al. 2003; Samish and Alexeev 2001). In addition, red imported fire ants (Solenopsis invictus) have been reported as predators of lone star tick nymphs and adults and are more prevalent in disturbed and burned areas (Harris and Burns 1972; Fleetwood et al. 1984).
Using Prescribed Fire Regimes to Manage Ticks
The frequency, intensity, and seasonality of a prescribed fire regime can affect ticks (Figure 5). Fire frequency refers to how many fires occur within a certain time. Fire intensity, often visibly estimated by the length of flames in the fire, refers to the rate or amount of heat or energy released. Longer flames indicate a more intense fire (Wade 2013). Fires that burn for a long time can have different effects than a rapidly moving fire. Fire seasonality refers to the timing of fire within a year. The effects of fire seasonality depend on weather and moisture conditions at the time of the fire as well as the species’ phenology, or seasonality of stages in the tick life cycle.

Credit: Raelene Crandall, UF/IFAS
Repeated burning causes lasting changes in vegetation structure. Some of these changes can be worse for ticks. A high fire frequency, or many fires within a short period, keeps landscapes open (Fill et al. 2022) and less hospitable for most of the five tick species, except perhaps the Gulf Coast tick (Gallagher et al. 2022). Changing the environment can decrease encounter rates of blacklegged and lone star ticks with white-tailed deer because there would be less vegetation from which to quest. Frequent fires also support the habitat structure favorable for tick predators such as northern bobwhite quail, which require fire return intervals of 18–30 months to reach large population sizes (TTRS 2020).
The effects of fire seasonality depend on the activity of ticks and their hosts. For example, late spring through summer is an active period for blacklegged tick larvae and nymphs and lone star tick adults and nymphs (Barbour and Fish 1993; White and Gaff 2018). Patchy burns during seasons of peak tick activity could also leave areas of refuge for ticks while at the same time meeting the varied needs of their hosts and predators across the landscape (Fill and Crandall 2018). Burning during the winter could decrease the survival of overwintering species such as American dog and blacklegged ticks but also have less effect on dormant plants, which could favor the rapid recovery of suitable habitat for these five tick species. Studies investigating the effects of fire size or patchiness on ticks have therefore produced mixed results (Hoch et al. 1972).
Although fire’s effects are complex, research suggests that burning southeastern landscapes regularly over the long term (1–4 year return intervals) can help control tick populations. To date, the tick populations controlled with fire have for the most part been lone star and blacklegged ticks. These species do not do well in dry open conditions (White and Gaff 2018), so clearing vegetation with fire has been a relatively effective control for them. There is evidence that the abundances of different stages of American dog ticks and blacklegged ticks can be reduced by fire within the same year (White and Gaff 2018). Gulf Coast ticks survive better in burned than in unburned habitats (Gleim et al. 2013), so it is uncertain how prescribed fire affects their populations directly in the long term. If you are considering using prescribed burns to control tick populations, consider what is known about the species, their habitats, and their hosts, and monitor the tick populations before and after fire to determine whether prescribed burning achieves the desired result. Methods for sampling tick abundances include trapping them and collecting them from host animals (see descriptions of potential methods: https://entnemdept.ufl.edu/creatures/urban/medical/deer_tick.htm).
Acknowledgements
The authors acknowledge funding for the fire science delivery program “The Southern Fire Exchange: Putting Fire Science on the Ground” from the Joint Fire Science Program and in agreement with the United States Forest Service, Southern Research Station. The Southern Fire Exchange supports the dissemination of and access to fire science information. To learn more, visit https://southernfireexchange.org/.
References
Allan, B. F. 2009. “Influence of Prescribed Burns on the Abundance of Amblyomma americanum (Acari: Ixodidae) in the Missouri Ozarks.” Journal of Medical Entomology 46 (5): 1030–1036. https://doi.org/10.1603/033.046.0509
Arsnoe, I. M., G. J. Hickling, H. S. Ginsberg, R. McElreath, and J. I. Tsao. 2015. “Different Populations of Blacklegged Tick Nymphs Exhibit Differences in Questing Behavior That Have Implications for Human Lyme Disease Risk.” PloS One 10 (5): e0127450. https://doi.org/10.1371/journal.pone.0127450
Barbour, A. G., and D. Fish. 1993. “The Biological and Social Phenomenon of Lyme Disease.” Science.” 260:1610–1616. https://doi.org/10.1126/science.8503006
Barnard, D. R.1986. “Density Perturbation in Populations of Amblyomma americanum (Acari: Ixodidae) in Beef Cattle Forage Areas in Response to Two Regimens of Vegetation Management.” Journal of Economic Entomology 79 (1): 122–127. https://doi.org/10.1093/jee/79.1.122
Burg, J. G. 2001. “Seasonal Activity and Spatial Distribution of Host-Seeking Adults of the Tick Dermacentor variabilis.” Medical and Veterinary Entomology 15:413–421. https://doi.org/10.1046/j.0269-283x.2001.00329.x
Burtis, J. C., J. B. Yavitt, T. J. Fahey, and R. S. Ostfeld. 2019. “Ticks as Soil-Dwelling Arthropods: An Intersection Between Disease and Soil Ecology.” Journal of Medical Entomology 56 (6): 1555–1564. https://doi.org/10.1093/jme/tjz116
Callaham, M. A, J. M. Blair, D. J. Kitchen, and M. R. Whiles. 2003. “Macroinvertebrates in North American Tallgrass Prairie Soils: Effects of Fire, Mowing, and Fertilization on Density and Biomass.” Soil Biology and Biochemistry 35 (8): 1079–1093. https://doi.org/10.1016/S0038-0717(03)00153-6
Center for Disease Control. 2020. “How Ticks Spread Disease” Available at: https://www.cdc.gov/ticks/life_cycle_and_hosts.html
Cilek, J. E., and M. A. Olson. 2000. “Seasonal Distribution and Abundance of Ticks (Acari: Ixodidae) in Northwestern Florida.” Journal of Medical Entomology 37 (3): 439–444. https://doi.org/10.1093/jmedent/37.3.439
Esteve‐Gassent, M. D., I. Castro‐Arellano, T. P. Feria‐Arroyo, R. Patino, A. Y. Li, R. F. Medina, A. A. P. de León, and R. I. Rodríguez‐Vivas. 2016. “Translating Ecology, Physiology, Biochemistry, and Population Genetics Research to Meet the Challenge of Tick and Tick‐Borne Diseases in North America.” Archives of Insect Biochemistry and Physiology 92 (1): 38–64. https://doi.org/10.1002/arch.21327
Fleetwood, S. C., P. D. Tee, and G. Thompson. 1984. “Impact of Imported Fire Ant on Lone Star Tick Mortality in Open and Canopied Pasture Habitats of East Central Texas.” Southwest Entomology 9:158–162.
Gallagher, M. R., J. K. Kreye, E. T. Machtinger, A. Everland, N. Schmidt, and N. S. Skowronksi. 2022. “Can restoration of fire-dependent ecosystems reduce ticks and tick-borne disease prevalence in the eastern United States?” Ecological Applications e2637. https://doi.org/10.1002/eap.2637
Ginsberg, H. S., G. J. Hickling, R. L. Burke, N. H. Ogden, L. Beati, R. A. LeBrun, et al. 2021. “Why Lyme Disease Is Common in the Northern US, but Rare in the South: The Roles of Host Choice, Host Seeking Behavior, and Tick Density.” PLoS Biology 19(1). https://doi.org/10.1371/journal.pbio.3001066
Gleim, E. R. 2020. “Lyme Disease Ecology: Working to Understand Lyme Ecology in a Newly Emerging Hot Spot & Implications for Beyond.” Webinar, North Atlantic Fire Science Exchange. Available at: https://www.youtube.com/watch?v=3PB8Or0Md5c
Gleim, E. R., L. M. Conner, and M. J. Yabsley. 2013. “The Effects of Solenopsis invicta (Hymenoptera: Formicidae) and Burned Habitat on the Survival of Amblyomma americanum (Acari: Ixodidae) and Amblyomma maculatum (Acari: Ixodidae).” Journal of Medical Entomology 50 (2): 270–276. https://doi.org/10.1603/ME12168
Gleim, E. R., L. M. Conner, R. D. Berghaus, M. L. Levin, G. E. Zemtsova, and M. J. Yabsley. 2014. “The Phenology of Ticks and the Effects of Long-Term Prescribed Burning on Tick Population Dynamics in Southwestern Georgia and Northwestern Florida.” PLoS One 9 (11): e112174. https://doi.org/10.1371/journal.pone.0112174
Gleim, E. R., G. E. Zemstova, R. D. Berghaus, M. L. Levin, M. Conner, and M. J. Yabsley. 2019. “Frequent Prescribed Fires Can Reduce Risk of Tick-Borne Diseases.” Scientific Reports 9:9974. https://doi.org/10.1038/s41598-019-46377-4
Greenberg, C. H., S. Miller, and T. A. Waldrop. 2007. “Short-Term Response of Shrews to Prescribed Fire and Mechanical Fuel Reduction in a Southern Appalachian Upland Hardwood Forest.” Forest Ecology and Management 243 (2): 231–236. https://doi.org/10.1016/j.foreco.2007.03.003
Hair, J. A., and D. E. Howell. 1970. “Lone Star Ticks: Their Biology and Control in Ozark Recreation Areas.” Oklahoma State University Agricultural Experiment Station, Bulletin B-679, Stillwater, OK.
Harris, W. G., and E. C. Burns. 1972. “Predation of the Lone Star Tick by the Imported Fire Ant.” Environmental Entomology 1:362–365. https://doi.org/10.1093/ee/1.3.362
Hoch, A. L., P. J. Semtner, R. W. Barker, and J. A. Hair. 1972. “Preliminary Observations on Controlled Burning for Lone Star Tick (Acarina: Ixodidae) Control in Woodlots.” Journal of Medical Entomology 9 (5): 446–451. https://doi.org/10.1093/jmedent/9.5.446
Ivey, T. L., and M. K. Causey. 1984. “Response of White-Tailed Deer to Prescribed Fire.” Wildlife Society Bulletin 12 (2): 138–141.
Jacobson, H. A., and G. A. Hurst. 1979. “Prevalence of Parasitism by Amblyomma americanum on Wild Turkey Poults as Influenced by Prescribed Burning.” Journal of Wildlife Diseases 15 (1): 43–47. https://doi.org/10.7589/0090-3558-15.1.43
Jones, D. D., L. M. Conner, T. H. Storey, and R. J. Warren. 2004. “Prescribed fire and raccoon use of longleaf pine forests: implications for managing nest predation?” Wildlife Society Bulletin 32 (4): 1255–1259. https://doi.org/10.2193/0091-7648(2004)032[1255:PFARUO]2.0.CO;2
Jorge, M. H., E. P. Garrison, L. M. Conner, and M. J. Cherry. 2020. “Fire and Land Cover Drive Predator Abundances in a Pyric Landscape.” Forest Ecology and Management 461:117939. https://doi.org/10.1016/j.foreco.2020.117939
Leonovich, S. A. 2015. “Ontogenesis of the Questing Behavior of Hard Ticks (Ixodidae).” Entomological Review 95 (6): 795–804. https://doi.org/10.1134/S0013873815060135
Mader, E. M., C. Ganser, A. Geiger, L. C. Harrington, J. Foley, R. L. Smith, N. Mateus-Pinilla, P. D. Teel, and R. J. Eisen. 2021. “A Survey of Tick Surveillance and Control Practices in the United States.” Journal Of Medical Entomology 1–10. https://doi.org/10.1093/jme/tjaa094
Mather, T. N., D. Fish, and R. T. Coughlin. 1994. “Competence of Dogs as Reservoirs for Lyme Disease Spirochetes (Borrelia burgdorferi).” Journal of the American Veterinarian Medical Association 205 (2): 186–188.
Mayo Clinic. 2021. “Slideshow: Guide to different tick species and the diseases they carry.” Available at: https://www.mayoclinic.org/tick-species/sls-20147911
Patterson T., and P. Knapp. 2018. “Longleaf Pine Masting, Northern Bobwhite Quail, and Tick-Borne Diseases in the Southeastern United States.” Applied Geography 98:1–8. https://doi.org/10.1016/j.apgeog.2018.06.010
Rosendale, A. J., M. E. Dunlevy, A. M. Fieler, D. W. Farrow, B. Davies, and J. B. Benoit. 2017. “Dehydration and Starvation Yield Energetic Consequences That Affect Survival of the American Dog Tick.” Journal of Insect Physiology 101:39–46. https://doi.org/10.1016/j.jinsphys.2017.06.012
Samish, M., and E. Aleskeeve. 2001. “Arthropods as Predators of Ticks (Ixodoidea).” Journal of Medical Entomology 38:1–11. https://doi.org/10.1603/0022-2585-38.1.1
Schulze, T. L., R. A. Jordan, and R. W. Hung. 1995. “Suppression of Subadult Ixodes scapularis (Acari: Ixodidae) Following Removal of Leaf Litter.” Journal of Medical Entomology 32 (5): 730–733. https://doi.org/10.1093/jmedent/32.5.730
Tall Timbers Game Bird Program. 2020. Bobwhite basics. Available at: https://talltimbers.org/game-bird-program-bobwhite-basics/
Teel, P. D., H. R. Ketchum, D. E. Mock, R. E. Wright, and O. F. Strey. 2010. “The Gulf Coast Tick: A Review of the Life History, Ecology, Distribution, and Emergence as an Arthropod of Medical and Veterinary Importance.” Journal of Medical Entomology 47 (5): 707–722. https://doi.org/10.1093/jmedent/47.5.707
Sonenshine, D. E. 2018. “Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease.” International Journal of Environmental Research and Public Health 15 (3): 478. https://doi.org/10.3390/ijerph15030478
Willis, D., R. Carter, C. Murdock, and B. Blair. 2012. “Relationship between Habitat Type, Fire Frequency, and Amblyomma americanum Populations in East‐Central Alabama.” Journal of Vector Ecology 37 (2): 373–381. https://doi.org/10.1111/j.1948-7134.2012.00241.x
White, A., and H. Gaff. 2018. “Application of Tick Control Technologies for Blacklegged, Lone Star, and American Dog Ticks.” Journal of Integrated Pest Management 9 (1): 12. https://doi.org/10.1093/jipm/pmy006
Wormser, G. P., D. McKenna, N. Piedmonte, V. Vinci, A. M. Egizi, B. Backenson, and R. C. Falco. 2020. “First Recognized Human Bite in the United States by the Asian Longhorned Tick, Haemaphysalis longicornis.” Clinical Infectious Diseases 70 (2): 314–316. https://doi.org/10.1093/cid/ciz449