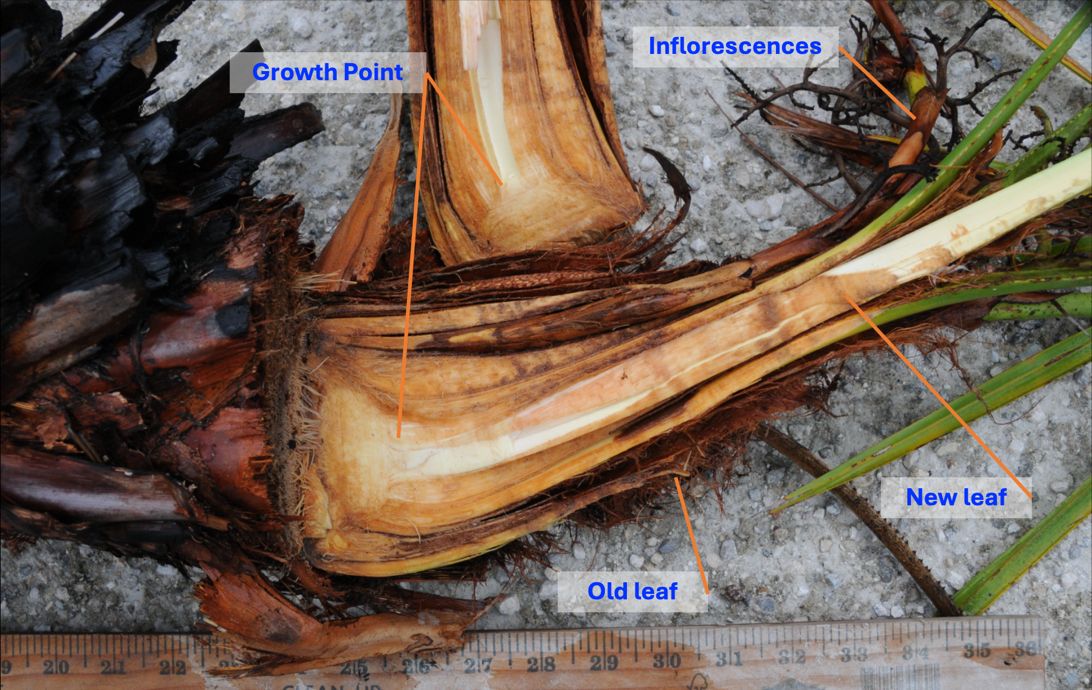
This publication informs fire managers, natural resources managers, and interested individuals about the relationship between saw palmetto and fire. Saw palmetto (Serenoa repens Bartr.) is a palm native to the southeastern United States and found in peninsular Florida, southern South Carolina, and southeastern Louisiana. It has two geographically segregated forms: the green form occurring inland and the silver form on Florida's east coast (Carrington and Mullahey 2006; Hilmon 1968; Maehr and Layne 1996). It occurs across many ecosystems that vary in soil moisture, such as mesic and scrubby flatwoods, mesic to xeric hammocks, xeric sandhills, coastal strands, dry prairies, and pine rocklands (FNAI 2010). Saw palmetto plants are low-growing bushes, ranging from 2 to 8 ft tall (0.6 to 2.4 m), with large, palmate, fan-shaped leaves (Gilman 2015). What appear to be stems are actually rhizomes, a specialized form of stems that grow near or below ground (Figure 1) (Hartmann et al. 2018). These rhizomes produce large flowering stalks, also called inflorescences, that can grow up to 3 ft (0.9 m) long (Gilman 2015).
Native Florida palms have fire-related traits, allowing them to persist in frequently burned environments (Abrahamson et al. 2023). Saw palmetto is a highly flammable species that comprises a significant part of Florida’s fire landscape (Foster and Schmalzer 2012). After fires, saw palmettos exhibit increased growth, resprouting, and flowering (Abrahamson 1999; Carrington et al. 2000; Hilmon 1968; Hilmon and Hughes 1965). Saw palmettos can survive fires because of their well-insulated buds hidden inside waxy leaves (Figure 2) (Maehr and Layne 1996). The meristem, where new growth originates, is located inside the top of the rhizome. Younger leaves grow closer to the center, and older leaves further out, like the layers of an onion (Fisher and Tomlinson 1973). The older leaves persist over time, providing insulation and protection to the meristem (Figure 2) (Henderson et al. 1995; Tomlinson 1990).

Credit: Vânia Pereira, UF/IFAS
Credit: Vânia Pereira, UF/IFAS
Fire Effects on Saw Palmetto
Carbohydrate reserves change.
Fires affect the carbohydrate reserves in saw palmettos. Following a winter burn in a long-unburned, mixed stand of slash pine and longleaf pine, saw palmettos showed a decrease in starch content and increased sugar levels. This suggests that saw palmetto uses sugars for plant regrowth. Four years after the fire, the starch and sugar levels returned to the levels found in unburned plants (Hough 1968). Saw palmetto resilience to fire and rapid resprouting has been associated with its extensive underground carbohydrate reserves stored in the rhizomes as well as with the extensive rhizome water content of 90% of fresh mass (fresh mass being the living, green plant tissue, as opposed to dead, dry leaves) (Abrahamson 1999; Diaz-Toribio and Putz 2021; Orzell et al. 2024).
Plant growth increases.
After fires, new leaves on saw palmetto plants are apparent within 7 to 14 days (Orzell et al. 2024). Within 1–2 years after the fire, plants that have been burned contain more than double the number of leaves of unburned plants. Burns occurring during the winter dry season prompt immediate leaf growth, which is typically slow during winter. This surge in new leaves significantly enhances the plants' photosynthetic capacity and their accumulation of carbohydrate reserves (Abrahamson and Abrahamson 2006). In oak-saw palmetto scrub environments, saw palmetto can return to its pre-burn height, over 1.64 feet (0.5 m), within one year, exhibiting high recovery rates and increased dominance (Schmalzer and Hinkle 1992). Fire also stimulates growth and vegetative reproduction by new sprouts, the primary method of plant spread. In other words, new individuals are formed along pre-existing rhizomes (Figure 3) (Hilmon 1968).

Credit: Vânia Pereira, UF/IFAS
Fire triggers flowering.
Saw palmetto typically flowers from March through May, independently of fire (Hilmon 1968). However, flowering is minimal in unburned areas where the overstory is dense (Abrahamson 1999). Fire during winter or summer seasons causes more plants to flower in the first year after fire than would flower without fire (Abrahamson 1999; Blonder et al. 2018; M. E. Carrington and J. J. Mullahey 2006; Carrington and Mullahey 2013). The production of flowers after a fire depends on plant size and light availability, as fire also reduces overstory canopy and increases light availability (Abrahamson 1999).

Credit: Vânia Pereira, UF/IFAS
Saw Palmetto Effects on Fire
Saw palmetto’s flammable leaves fuel fire.
Fires are fueled by saw palmetto’s highly combustible dead leaves (Carrington et al. 2000). With its high biomass of live and dead foliage, saw palmetto creates a greater wildfire hazard than other shrubs and small trees growing between the forest canopy and the forest floor (Behm et al. 2004). Although the leaves do not reach high temperatures during a fire, leaf consumption is high (Figure 4), and the time to ignition is shorter than that of other common Florida understory species. This results from the higher litter depth typically found under saw palmetto plants, leaves positioned closer to the litter surface, and lower foliar moisture content (Figure 5) (Behm et al. 2004). The low fuel moisture in dead leaves, even after a short-term rainfall, has been associated with the position of the dead leaves. Dead leaves lie under living leaves, which have a waxy coating that prevents rain from wetting the dead leaves (Figure 5) (Hiers et al. 2019). Dense patches of saw palmetto can, therefore, greatly increase fire intensity (Platt 1999).

Credit: Vânia Pereira, UF/IFAS
Saw Palmetto and Fire: Best Management Practices
Control fuels.
In long-unburned areas with high fuel loads, managers commonly manipulate fuels to reduce fire hazards and enhance the safety of prescribed burning. Mechanical mastication, the mowing, shredding, or chipping of understory plants such as saw palmetto, is often used to restructure the understory fuel height. This practice does not reduce fuel loads but alters and rearranges the structure of the fuel bed, decreasing its height and increasing its density (Kreye et al. 2015). A lower, denser bed of understory fuel has been shown to decrease flame heights by 66%. When using mastication, it’s advised to ignite prescribed burns soon afterward and to choose a time when the soil moisture is high. Wet soils reduce the likelihood of high soil temperatures during burns. Greater fuel bed density can increase soil temperatures, which could hurt or kill nearby plants, namely pine trees, especially if the soil is dry (Kreye and Kobziar 2015). The loss of shrub cover on longleaf pine flatwoods by mowing has been shown to increase the drying of surface fuels, whereas the increase in bulk density can increase the ignition probability (Budny et al. 2014).
Due to the rapid regrowth of shrubs, such as saw palmetto, following disturbances, fuel restructuring is only effective in the short term. After mastication, the post-treatment fuel reduction burns should occur within six months (Kreye and Kobziar 2015; Kreye et al. 2014). For fuel control in longleaf pine flatwoods, follow-up prescribed burning after mowing should occur within one year (Budny et al. 2014). Prescribed fire alone is also an effective management practice to control saw palmetto, but it needs to be applied recurrently every 3–5 years in mesic pine savannas (Brose and Wade 2002). If mastication kills saw palmettos, the loss will likely be long-term. Saw palmetto rarely reestablishes via seeds.
Effective management of high fuel loads is crucial to prevent severe wildfires, which can kill plants, including saw palmetto. In 2013, an intense fire spread through long-unburned sand pine scrub, an area that had remained fire-free for eight decades. The temperatures reached during this fire exceeded the melting point of aluminum (660°C or 1220°F), with retention times that caused aluminum research tags to melt. Seven smaller saw palmetto plants died due to the intense heat. The saw palmetto mortality was linked to several factors, including the high fuel load, dense shading of the plants before the fire, and the size of the plants, which were smaller with limited reserves for resprouting. The loss of saw palmetto highlights the importance of implementing prescribed burning as part of fire management strategies to prevent severe fires and preserve plant species (Abrahamson et al. 2023).
Schedule burns to aid flower and fruit production.
Saw palmetto fruits are widely used as phytotherapy for prostate cancer prevention in European and American markets. Due to the high demand for fruits in medicinal markets, interest in the supply chain has increased over the years, and saw palmetto’s relationship with fire has been a major area of research.
In central and southwest Florida, Carrington and Mullahey (2006) found that the timing and frequency of burns affect how much saw palmetto flowers after fire. The percentage of plants in the study area producing flowering stalks was greater in the period after fire. After burns during the growing seasons (April to July), saw palmetto plants had a 50% flowering rate one year later. However, only 15% of plants flowered in the second year after fire. For burns during the winter (November to February), about 21% and 28% of plants flowered in the first and second years after the fire, respectively. Saw palmetto flowers in April and May, around 9 to 12 months after growing season burns and 2 to 5 months after winter burns (Carrington and Mullahey 2006). Researchers examined whether fire frequency affects the amount of flowering after summer burns. They found that in pine flat woods and dry prairies of Florida, burning every five years promotes more flowering and fruiting than every 2–3 years. It should be noted, however, that this study did not examine winter burns (Carrington and Mullahey 2013).
The flowering of saw palmetto can be influenced by factors other than the seasonality and frequency of fires. For instance, flowering can vary across ecosystems. Abrahamson (1999) examined flowering patterns in multiple ecosystems, from xeric scrub to xeric sandhill and mesic flatwoods along the Lake Wales Ridge. He found that the flowering of saw palmetto was affected by burn season (January to July fires) and the frequency of burns (three in 8 years). It was additionally affected by plant size, crown loss to fire, and light availability (Abrahamson 1999). The increase in flowering after fires, however, did not always translate to high fruit production. Saw palmetto is notoriously difficult to predict, and the harvest of saw palmetto fruits is highly variable. Fruiting can be inhibited by a multitude of external factors such as caterpillar predation (Litoprosopus futilis Grote and Robinson) and fungal infection (Colletotrichum gloeosporioides (Penz.) Penz. and Sacc.) (Carrington et al. 2001).
Did you know?
- This long-lived palm is estimated to persist for 10,000 years or longer (Takahashi et al. 2011).
- Saw palmetto is an ornamental plant and a source of food for Florida wildlife. It is used for many products, including honey, fiber, oil, wax, roof thatch, medicines, and medicines (Bennett and Hicklin 1998).
- Over 300 insect species have been observed visiting saw palmetto flowers (Deyrup and Deyrup 2012).
- Saw palmetto is a crucial cover for Florida panthers and black bears when they give birth to their young. After birth, mammal offspring use the plants for food and shelter (Maehr and Layne 1996).
- Saw palmetto is predicted to stay within its current range despite a slight increase of suitable habitat to the northeast of its US range (based on climate change predictions to 2070) (Butler and Larson 2020).

Credit: Vânia Pereira, UF/IFAS
References
Abrahamson, W. 1999. "Episodic Reproduction in Two Fire-Prone Palms, Serenoa repens and Sabal etonia (Palmae)." Ecology 80 (1): 100–115.
Abrahamson, W. G., and C. R. Abrahamson. 2006. Post-Fire Canopy Recovery in Two Fire-Adapted Palms, Serenoa repens and Sabal etonia (arecaceae). Florida scientist 69 (2): 69–79.
Abrahamson, W. G., C. R. Abrahamson, S. M. Koontz, E. H. Tran, E. S. Menges, and A. S. David. 2023. "What kills the virtually immortal palms of the Florida scrub?" American Journal of Botany 110 (10): 1–e16234. https://doi.org/10.1002/ajb2.16234
Behm, A. L., M. L. Duryea, A. J. Long, and W. C. Zipperer. 2004. "Flammability of Native Understory Species in Pine Flatwood and Hardwood Hammock Ecosystems and Implications for the Wildland-Urban Interface. International Journal of Wildland Fire 13 (3): 355. https://doi.org/10.1071/WF03075
Bennett, B., and J. Hicklin. 1998. "Uses of Saw Palmetto (Serenoa repens, Arecaceae) in Florida." Economic Botany 52 (4): 381–393. https://doi.org/10.1007/BF02862068
Blonder, B. I., J. M. Wooldridge, and M. B. Garrard. 2018. "Assessing Postfire Vegetative Changes and Implications for Management in a Northeast Florida Coastal Strand Ecosystem." Castanea 83 (1): 104–121. https://doi.org/10.2179/17-127
Brose, P., and D. Wade. 2002. "Potential Fire Behavior in Pine Flatwood Forests following Three Different Fuel Reduction Techniques." Forest Ecology and Management 163 (1): 71–84. https://doi.org/10.1016/S0378-1127(01)00528-X
Budny, M. L., J. K. Kreye, L. N. Kobziar, and J. M. Camp. 2014. Fuel Treatments in Pine Flatwoods: A Photo Series Guide. University of Florida, School of Forest Resources and Conservation, Kobziar Fire Science Lab, and . Southern Fire Exchange. May–June. https://southernfireexchange.org/wp-content/uploads/Fuel_Treatments_Photo_Guide.pdf
Butler, C. J., and M. Larson. 2020. "Climate Change Winners and losers: The Effects of Climate Change on Five Palm Species in the Southeastern United States." Ecology and Evolution 10 (19): 10408–10425. https://doi.org/10.1002/ece3.6697
Carrington, M. E., and J. J. Mullahey. 2006. "Effects of Burning Season and Frequency on Saw Palmetto ( Serenoa repens) Flowering and Fruiting." Forest Ecology and Management 230 (1): 69–78. https://doi.org/10.1016/j.foreco.2006.04.020
Carrington, M. E., and J. J. Mullahey. 2013. "Saw Palmetto (Serenoa repens) Flowering and Fruiting Response to Time Since Fire." Rangeland Ecology and Management 66 (1): 43–50. https://doi.org/10.2111/REM-D-11-00183.1
Carrington, M. E., J. J. Mullahey, G. Krewer, B. Boland, and J. Affolter. 2000. "Saw Palmetto (Serenoa repens): An Emerging Forest Resource in the Southeastern United States." Southern Journal of Applied Forestry 24 (3): 129–134. https://doi.org/10.1093/sjaf/24.3.129
Carrington, M. E., P. D. Roberts, N. V. R. R. Urs, R. J. McGovern, T. E. Seijo, and J. J. Mullahey. 2001. "Premature Fruit Drop in Saw Palmettos Caused by Colletotrichum gloeosporioides." Plant Disease 85 (2): 122–125. https://doi.org/10.1094/PDIS.2001.85.2.122
Deyrup, M., and L. Deyrup. 2012. "The Diversity of Insects Visiting Flowers of Saw Palmetto (Arecaceae)." The Florida Entomologist 95 (3): 711–730. https://doi.org/10.1653/024.095.0322
Diaz-Toribio, M. H., and F. E. Putz. 2021. "Underground Carbohydrate Stores and Storage Organs in Fire-Maintained Longleaf Pine Savannas in Florida, USA." American Journal of Botany 108 (3): 432–442. https://doi.org/10.1002/ajb2.1620
Fisher, F. B., and P. B. Tomlinson. 1973. "Branch and Inflorescence Production in Saw Palmetto (Serenoa repens)." Principes- Journal of The Palm Society 17 (1): 10–19.
FNAI, F. N. A. I. 2010. Guide to the Natural Communities of Florida.
Foster, T. E., and P. A. Schmalzer. 2012. "Growth of Serenoa repens Planted in a Former Agricultural Site." Southeastern Naturalist 11 (2): 331–336. https://doi.org/10.1656/058.011.0214
Hartmann, H., D. Kester, F. Davies, R. Geneve, and S. Wilson. 2018. Hartmann and Kester's Plant Propagation: Principles and Practices (Vol. 9). Pearson.
Henderson, A., G. Galeano, and R. Bernal. 1995. Field Guide to the Palms of the Americas. Princeton University Press.
Hiers, J. K., C. L. Stauhammer, J. J. O’Brien, H. L. Gholz, T. A. Martin, J. Hom, and G. Starr. 2019. "Fine Dead Fuel Moisture Shows Complex Lagged Responses to Environmental Conditions in a Saw Palmetto (Serenoa repens) Flatwoods." Agricultural and Forest Meteorology 266–267:20–28. https://doi.org/10.1016/j.agrformet.2018.11.038
Hilmon, J. B. 1968. Autecology of Saw Palmetto (Serenoa repens (Bartr.) Small) ThesisDuke University.].
Hough, W. A. 1968. "Carbohydrate Reserves of Saw-Palmetto: Seasonal Variation and Effects of Burning." Forest Science 14 (4): 399–405.
Kreye, J., D. Godwin, and L. Kobziar. 2015. "Mechanical Treatments in Pine Flatwoods: A Temporary Rearrangement of Fuel Structure." Southern Fire Exchange. https://southernfireexchange.org/wp-content/uploads/2015-1.pdf
Kreye, J. K., and L. N. Kobziar. 2015. "The Effect of Mastication on Surface Fire Behaviour, Fuels Consumption and Tree Mortality in Pine Flatwoods of Florida, USA." International Journal of Wildland Fire 24 (4): 573–579. https://doi.org/10.1071/WF14186
Kreye, J. K., L. N. Kobziar, and J. M. Camp. 2014. "Immediate and Short-Term Response of Understory Fuels following Mechanical Mastication in a Pine Flatwoods Site of Florida, USA." Forest Ecology and Management 313:340–354. https://doi.org/10.1016/j.foreco.2013.10.034
Maehr, D. S., and J. N. Layne. 1996. "Florida's All-Purpose Plant: the Saw Palmetto." The Palmetto 16 (4): 6–10, 15, 21.
Orzell, S. L., M. Bitomský, E. L. Bridges, B. Budach, J. Klimešová, J. Martinková, Z. E. Reed, and S. J. Raynor. 2024. "Florida’s Fiery Subtropical Grasslands: Growth Forms, Belowground Organs, and Post-Fire Recovery Strategies." Folia geobotanica. https://doi.org/10.1007/s12224-024-09440-1
Platt, W. J. 1999. "Southeastern Pine Savannas." The Savanna, Barren, and Rock Outcrop Communities of North America 23–51.
Schmalzer, P. A., and C. R. Hinkle. 1992. "Recovery of Oak: Saw Palmetto Scrub after Fire. Castanea 57 (3),: 158–173.
Takahashi, M. K., L. M. Horner, T. Kubota, N. A. Keller, and W. G. Abrahamson. 2011. "Extensive Clonal Spread and Extreme Longevity in Saw Palmetto, a Foundation Clonal Plant." Molecular ecology 20 (18):, 3730–3742. https://doi.org/10.1111/j.1365-294X.2011.05212.x
Tomlinson, P. B. 1990. The Structural Biology of Palms. Clarendon Press.