Nutrient management is closely associated with fertilizer type, application rate, application time, and application placement. For example, blueberry plants prefer ammoniacal nitrogen rather than nitrate nitrogen for their growth and development. However, most crops use both ammoniacal and nitrate nitrogen. Proper nutrient management should include the “Four R’s” of fertilizer use: apply the right nutrient, at the right rate, at the right time, and in the right place for the selected crop (Mikkelsen 2011). In Florida, there is another R, i.e., the 5th R, the right irrigation, please read https://edis.ifas.ufl.edu/publication/CV296 for more details (Liu et al. 2020). This article focuses on how to select the right fertilizer to enhance profitability and satisfy best management practices (BMPs). There are many fertilizer sources available for commercial crop production. The characteristics of each fertilizer type determine whether its use poses an advantage or a disadvantage to a farmer. This article provides a general overview on quick- and slow-release fertilizers for commercial crop producers, crop consultants, crop advisers, UF/IFAS Extension faculty, researchers, and students who are interested in nutrient management for commercial crop production.
Most used commercial fertilizers are water-soluble quick-release fertilizers (QRFs) that are predictively readily available for plants when properly placed in soil. Quick-release fertilizers are ideal for pre-plant applications, side dressing, hydroponics, or fertigation for many crops, including vegetables. They are highly practical if nutrient leaching or immobilization of nutrients by soil particles is not a serious concern (Wolf 1999), especially if unpredictable, high-leaching/flooding events do not occur. If conditions are favorable, less expensive QRFs have proven to be effective in crop production.
In the best conditions, QRFs become available to plants at a consistent rate (Trenkel 2010). They will release all readily available nutrients in a short period of time after being properly applied to soil with appropriate soil moisture. In other words, their release curve is immediate and does not synchronize with or match the dynamic needs of crop growth, which is why applying timely side dressings is necessary. In fact, crop nutrient requirements change as plants develop. For example, snap bean (Figure 1) has a slow-fast-slow growth stage pattern that has a smaller nutrient requirement in the early stage, a greater nutrient requirement in the middle stage, and a smaller nutrient requirement again in the late stage. Traditionally, multiple fertilizer applications, or side dressings, have been used to accommodate plant nutrient demand, minimize nutrient losses, and increase fertilizer use efficiency. Using too much fertilizer all at once without timing applications to the plants' growth stage pattern can expose plants to burning and cause nutrient loss through leaching or runoff. It frequently means that nutrients will not be available to plants when they need them. To deal with these challenges, the global fertilizer industry has been working to develop new fertilizers called controlled-release fertilizers (CRFs) and slow-release fertilizers (SRFs). These fertilizers have become more and more popular in recent years (Robbins 2005).
Controlled-Release Fertilizers
The Association of American Plant Food Control Officials defines CRFs as fertilizers that contain a plant nutrient in a form the plant cannot immediately absorb. Uptake is delayed after application, so that CRFs provide the plant with available nutrients for a longer time compared to QRFs, such as urea.
Controlled-release fertilizers are typically coated or encapsulated with inorganic or organic materials that control the rate, pattern, and duration of plant nutrient release. Polymer-coated urea exemplifies CRFs (Du et al. 2006; Loper and Shober 2012). These fertilizers control the release of nutrients with semi-permeable coatings, occlusion, protein materials, or other chemical forms, by slow hydrolysis of water-soluble, low-molecular-weight compounds, or by other unknown means (Trenkel 2010). Most importantly, the release rate of a CRF fertilizer is designed in a pattern synchronized to meet changing crop nutrient requirements.
As required by Florida rule, at soil temperatures below 77°F, a CRF must meet the following three criteria: (1) less than 15 percent of the CRF nutrients should be released in 24 hours, (2) less than 75 percent should be released in 28 days, and (3) at least 75 percent should be released by the stated release time (40–360 days) (Trenkel 1997).
Slow-Release Fertilizers
Nitrogen products decomposed by microbes are commonly referred as SRF fertilizers. Some SRFs such as N-SURE are made in factories. However, some such as manure are naturally originated and cannot be formulated to permit controlled release (Liu et al. 2011). The nutrient release pattern of SRFs is fully dependent on soil and climatic conditions. Slow-release fertilizer releases nutrients gradually with time, and it can be an inorganic or organic form. An SRF contains a plant nutrient in a form that makes it unavailable for plant uptake and use for some time after the fertilizer is applied. Such a fertilizer extends its bioavailability significantly longer than QRFs such as ammonium nitrate, urea, ammonium phosphate, or potassium chloride.
Nitroform (also referred to as trinitromethane with a chemical formula HC[NO2]3) exemplifies inorganic SRF fertilizers (Loper and Shober 2012). Urea-formaldehyde (UF), urea-isobutyraldehyde/isobutylidene diurea (IBDU), and urea-alcetaldehyde/cyclo diurea (CDU) typify organic SRF fertilizers (Trenkel 2010). Based on the source, there are two types of SRF fertilizers: natural and artificial (Table 1).
Natural SRFs include plant manures, such as green manure or cover crops, all animal manures (chicken, cow, and poultry) and compost (Shukla et al. 2013). Because of their organic nature, these must be broken down by microbial activity before the nutrients can be released to crops. In general, organic fertilizers may take a long time to release nutrients, and these nutrients may not be available when the plant needs them. The duration of nutrient release of this type of organic fertilizers mainly depends on soil microbial activity that is driven by soil moisture and temperature. Organic SRFs contain both macro-nutrients (nitrogen, phosphorus, potassium, etc.) and micro-nutrients (iron, manganese, copper, etc). The nutrient concentrations of organic SRFs are relatively lower than those of synthetic SRF fertilizers. For example, Sup'r Green brand is a chicken manure fertilizer containing only 3-2-2 % N, P2O5, and K2O, respectively.
Synthetic SRFs are sparingly water-soluble. The bioavailability of this type of fertilizers (typically in pellet or spike form) depends on soil moisture and temperature. Nutrients are released throughout a period of time that may range from 20 days to 18 months (Trenkel 2010). Therefore, fewer applications are needed with SRFs, but nutrients are released based upon the temperature and moisture conditions in the soil, which may not match the crop growth demand due to varying weather conditions (Trenkel 2010). Synthetic SRFs often contain a single nutrient at a much higher level than would occur in a natural SRF. For example, N-Sure® is a SRF that contains 28 percent nitrogen (28-0-0) (Clapp 1993; Liu and Williamson 2013)
The Difference between Slow- and Controlled-Release Fertilizers
- The terms "slow-release fertilizer," or SRF, and "controlled-release fertilizer," or CRF, do not mean the same thing.
- Controlled-release fertilizer is also known as controlled-availability fertilizer, delayed-release fertilizer, metered-release fertilizer, coated fertilizer (Oertli and Lunt 1962), or slow-acting fertilizer (Gregorich et al. 2001). According to Shaviv (2005), "The term controlled-release fertilizer became acceptable when applied to fertilizers in which the factors dominating the rate, pattern and duration of release are well known and controllable during CRF preparation."
- Slow-release fertilizers involve a slower release rate of nutrients than conventional water-soluble fertilizers, but the rate, pattern, and duration of release are not controlled (Trenkel 2010) because they depend on microbial organisms whose effectiveness is dependent on soil temperature and moisture conditions.
- Because of their dependence on microbial digestion to enable nutrient availability, SRFs occasionally pose the risk of increased harmful leaching events. This situation occurs when favorable conditions for microbial activity follow after the cropping cycle. Excess available nutrients can be pollutants irrespective of the source.
Advantages of Using CRFs and SRFs
The major advantages for using SRFs or CRFs include:
- Decreased nutrient losses and enhanced nutrient-use efficiency. The application of CRFs and SRFs can potentially decrease fertilizer use by 20 to 30 percent of the recommended rate of a conventional fertilizer while obtaining the same yield (Trenkel 2010).
- Minimization of fertilizer-associated risks such as leaf burning, water contamination, and eutrophication (a process where water bodies receive excess nutrients). The slow rates of nutrient release can keep available nutrient concentrations in soil solution at a lower level, reducing runoff and leaching losses.
- Reduced application and labor costs. For example, in current practices, commercial potato producers use 3 to 4 applications of nitrogen fertilizers for northeast Florida and 2 applications for southwest Florida (personal communication with local potato producers). Eliminating extra applications of fertilizer saves the farmer between $5 and $7/acre broadcasting expense (Liu et al. 2011). Additionally, avoidance of fertilizer application in late growth stage eliminates plant damages to crops.
- Better understanding of nutrient release rate and duration (CRFs only, because they are less sensitive to soil and climate conditions) (Shaviv 2005; Shoji 2005; Trenkel 2010). Knowing when to apply fertilizer and in what quantities saves money, reduces fertilizer-associated risks to crops and the environment, and improves nutrient management programs.
- Lowered soil pH in alkaline soils for better bioavailability of some nutrients. Applying sulfur-coated urea will probably increase soil acidity because both sulfur and urea contribute to increasing the acidity (lowering soil pH) of the soil. Consequently, phosphorus or iron may be more bioavailable and benefit some crops like blueberry, potato, and sweet potato (Liu and Hanlon 2012). In addition, sulfur is an essential nutrient for all crops.
- Reduced production costs if there is an abundant supply of SRF sources like manures nearby.
Disadvantages of Using CRFs and SRFs
- Most coated or encapsulated CRFs and SRFs (Tables 1 & 2) cost considerably more to manufacture than conventional fertilizers. This extra cost increases growers' crop production costs. For example, the price was $650 per ton for environmentally smart nitrogen (ESN) (44% N) versus $481 per ton for urea (46 percent N) (Ruark 2012). Environmentally smart nitrogen was 35.1 percent more costly than urea. The price per unit of nitrogen was 41.3 percent greater for ESN than for conventional urea.
- Applying sulfur-coated urea almost always lowers soil pH as aforementioned. However, this acidification may cause nutrient disorders such as calcium deficiency or magnesium deficiency if there is not a proper nutrient management program.
- Nutrient deficiencies may occur if nutrients are not released as predicted because of low temperatures, flooded or droughty soil, or poor activity of soil microbes.
- Possible uncontrolled nutrient release of SRFs. Use efficiency of SRFs may be enhanced by planting shelter belts or nutrient trap crops where runoff is likely to occur.
How are CRFs or SRFs best used?
Crop nutrient requirements follow a dynamic pattern: they begin low in the early growth stage, increase sharply in the middle stage and decrease in the late stage (Figure 1). Conventional fertilizers (QRFs) are instantly available when they're applied, which makes them more vulnerable to loss from a variety of causes such as ammonia volatilization (ammonia emitting into the atmosphere), de-nitrification (nitrate is reduced to nitrogen), leaching, or runoff after being applied to the soil. Nitrogen and potash fertilizers are particularly easily lost. Assuming the same amount of fertilizer is applied, a one-time or seasonal application of conventional fertilizers has the potential to lose much more nitrogen than would be lost with multiple split-applications of fertilizer (Figure 2). Split applications of convention fertilizer are recommended (Hochmuth and Hanlon 2013).
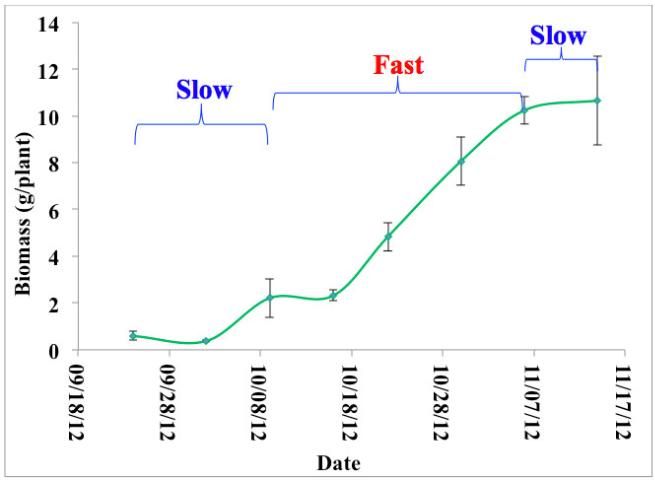
Credit: Guodong Liu, UF/IFAS
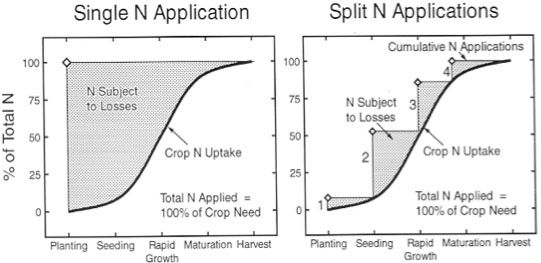
Credit: Waskom, Cardon, and Crookston
To match crop nutrient requirements, the ideal fertilizer should have this characteristic: the nutrient release matches the nutrient requirements of the crop throughout all of the plant growth stages (Figure 3). Obviously, QRFs do not have this characteristic, and they cannot meet such requirements without repeat applications. Fortunately, using deliberate applications of CRFs and SRFs in specific circumstances where they are appropriate can accommodate timely plant nutrient demand requirements, maximize nutrient use efficiency, and minimize environmental concerns. There is a close relationship between CRFs and BMPs. Section 32 of Water Quality/Quantity Best Management Practices for Florida Vegetable and Agronomic Crops (2005 edition) discusses CRFs and BMPs including planning and application of CRFs.
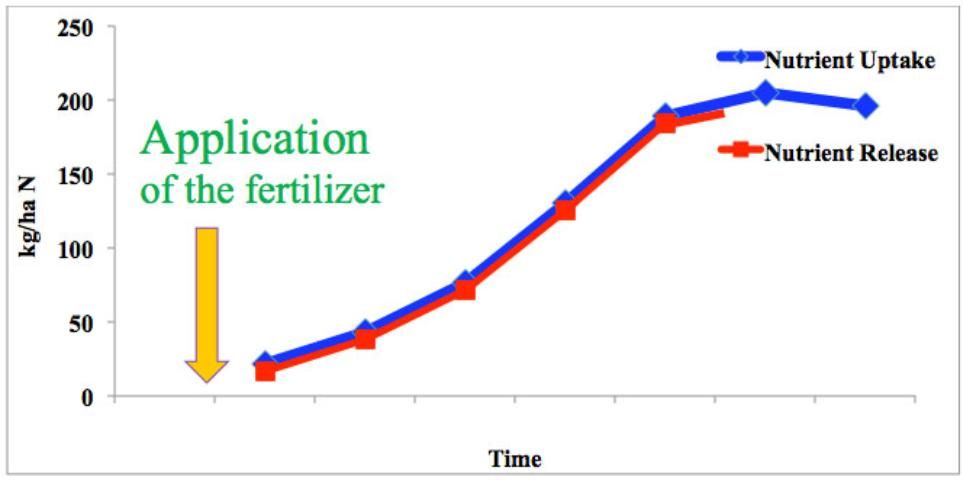
Credit: Adapted from Lammel 2005
Take-Home Message
- Quick-release fertilizers are water soluble and readily available for plants to take up when they are properly placed at the right time.
- Controlled-release fertilizers contain a plant nutrient in a form that delays its availability for plant uptake and use after application, or that extends its availability to the plant significantly longer than "rapidly available fertilizers" such as ammonium nitrate or urea, ammonium phosphate, and potassium chloride.
- Controlled-release fertilizers can dynamically release nutrients and meet the crop's changing nutrient demand throughout its growth cycle, maximize nutrient use efficiency, and minimize environmental concerns.
- Slow-release fertilizers generally have a slower release rate of the nutrient than conventional water-soluble fertilizers and CRFs. However, the rate, pattern and duration of release are not well controlled because they are dependent on microbial activity that is driven by soil moisture and temperature conditions. Slow-release fertilizers can occasionally be released very quickly when excessive moisture and high temperatures occur in the same period.
- Use of CRFs or SRFs can reduce nutrient losses, increase nutrient-use efficiency, and protect the environment. Thus, the application of CRFs or SRFs is a Best Management Practice (BMP) tool for crop production.
References
Clapp, J.P., Jr. 1993. "Foliar application of liquid urea-triazone-based nitrogen fertilizers and crop safety." 3(4): 442–444.
Du, C., J. Zhou, A. Shaviv. 2006. Release Characteristics of Nutrients from Polymer-coated Compound Controlled Release Fertilizers. Journal of Polymers and the Environment 14 (3): 223–230. https://link.springer.com/article/10.1007/s10924-006-0025-4
Gregorich, E. G., L. W. Turchenek, M. R. Carter, and D. A. Angers. 2001. Soil and Environmental Science Dictionary. CRC Press. p. 132. ISBN 978-0-8493-3115-2. LCCN 2001 S592.S49 2001. Retrieved 30 December 2013.
Lammel, J. 2005. "Cost of the different options available to the farmers: Current situation and prospects." IFA International Workshop on Enhanced-Efficiency Fertilizers. Frankfurt. International Fertilizer Industry Association, Paris, France.
Liu, G., E. H. Simonne, K. T. Morgan, G. J. Hochmuth, S. Agehara, and R. Mylavarapu. 2020. 2020–2021 Vegetable Production Handbook: Chapter 2. Fertilizer Management for Vegetable Production in Florida. CV296. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/CV296
Loper, S. and A. L. Shober. 2012. Soils & Fertilizers for Master Gardeners: Glossary of Soil and Fertilizer Terms. SL 277. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/MG457
Oertli, J. J. and J. Lunt. 1962. "Controlled release of fertilizer minerals by encapsulating membranes: I. Factors influencing the rate of release." Soil Sci. Soc. Proc. 26: 579–583.
Robbins, J. "Slow-release fertilizers as tools." IFA International Workshop on Enhanced-Efficiency Fertilizers. Frankfurt, Germany, 28–39 June 2005.
Ruark, M. 2012. "Advantages and disadvantages of controlled-release fertilizers." https://soilsextension.webhosting.cals.wisc.edu/wp-content/uploads/sites/68/2014/02/Overview_of_fertilizer_technologies_2012_WIFFVC.pdf
Shaviv, A. 2000. "Advances in controlled release of fertilizers." 71: 1–49.
Shukla, S., E. A. Hanlon, F. H. Jaber, P. J. Stoffella, T. A. Obreza, and M. Ozores-Hampton. 2013. Groundwater Nitrogen: Behavior in Flatwoods and Gravel Soils Using Organic Amendments for Vegetable Production. CIR 1494. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/AE400
Trenkel, M. E. 2010. "Slow- and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture." International Fertilizer Industry Association (IFA) Paris, France.
Waskom, R. M., G. E. Cardon, and M. A. Crookston. 1994. "Best Management Practices for Irrigated Agriculture." Colorado Water Resources Institute Report No. 184.
Wolf, B.. 1999. The Fertile Triangle: The Interrelationship of Air, Water, and Nutrients in Maximizing Soil Productivity. Binghamton, NY: The Haworth Press, Inc.