The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids, and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The citrus snow scale, Unaspis citri Comstock, is an armored scale belonging to the family Diaspididae, which is comprised of 2400 species of armored scales. The family Diaspididae constitutes one of the most successful groups of plant-parasitic arthropods and includes some of the most damaging and unmanageable pests of perennial crops and ornamentals (Beardsley and Gonzalez 1975). Citrus snow scale is considered to be a pest on citrus species and has been reported on all varieties, with mandarins being the least favorable (Dickens 1968).

Credit: J. L. Castner, UF/IFAS
Citrus has been commercially farmed since the mid-1800s in Florida, but the citrus snow scale did not become a problem until grove expansion significantly increased in the 1960s. The freeze of 1962 was the main factor that increased the spread of citrus snow scale across the state because so many trees were damaged. In response to this devastating damage, many growers replaced the damaged trees with new saplings from nurseries. These new citrus trees were already infested with the citrus snow scale, and once transplanted within the new groves it spread to already established trees.
Although citrus snow scale may be commonly found in Florida citrus groves, populations today rarely result in economic damage. Several historical citrus surveys conducted by the Florida Department of Agricultural Services-Division of Plant Industry (FDACS-DPI) indicated that citrus snow scale is quite common within Florida (Hodges 2013). Current citrus sample submissions for FDACS-DPI focus on more recent invasive pests of concern such as the Asian citrus psyllid, Diaphorina citri, and the accompanying citrus greening disease transmitted by the psyllid (Stocks 2013).

Credit: Jeffrey W. Lotz, FDACS-Division of Plant Industry
Synonymy
Chionaspis citri Comstock
Howardia citri Comstock
Prontaspis citri Comstock
Dinaspis veitchi Green & Laing
Trichomytilus veitchi Green & Laing
Dinaspis annae Malenotti
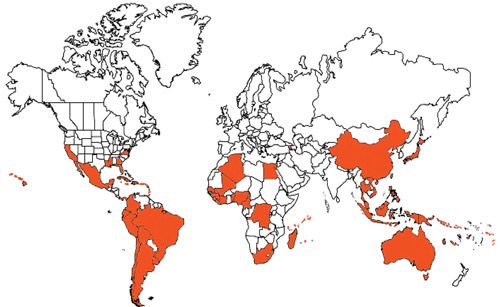
Distribution
Citrus snow scale is thought to have originated from Asia, but it is now found in many citrus-growing regions around the world, including:
Afrotropical: Benin, Cameroon, Comoros, Côte d'Ivoire, Guinea, Liberia, Madagascar, Mali, Mauritius, Nigeria, Senegal, Seychelles, Sierra Leone, South Africa, Togo, and Zaire.
Australasian: American Samoa, Australia, Queensland, Cook Islands, Federated States of Micronesia, Truk Islands, Fiji, Hawaiian Islands, Kiribati, New Caledonia, Niue, Papua New Guinea, Solomon Islands, Tonga, Vanuatu, Wallis and Futuna Islands, and Western Samoa.
Nearctic: Mexico, Veracruz, and the United States of America (California, Florida, Georgia, Louisiana, Mississippi, Virginia).
Neotropical: Antigua and Barbuda, Argentina, Barbados, Bermuda, Bolivia, Brazil, Mato Grosso, Rio Grande do Sul, Rio de Janeiro, Sao Paulo, Chile, Colombia, Cuba, Dominican Republic, Ecuador, El Salvador, Grenada, Jamaica, Netherlands Antilles, Panama Canal Zone, Peru, Puerto Rico and Vieques Island, Saint Croix, Saint Lucia, Trinidad and Tobago, and Uruguay.
Oriental: China, Guangxi, Hainan, Hubei, Sichuan, Zhejiang, Hong Kong, Indonesia, Kalimantan, Malaysia, Philippines, Mindanao, Singapore, Taiwan, Thailand, Japan, and Vietnam.
Palaearctic: Algeria, Armenia, Azores, and Egypt.
Credit: Courtney R. Buckley, University of Florida, Entomology and Nematology Department
Life Cycle and Biology
Armored scales are distinguishable from soft scales by the removable cover or 'armor'. As crawlers, the females of this family insert their long mouthparts into suitable hosts and never move again (Fasulo and Brooks 2010, Miller and Davidson 2005). The immature males become immobile once they begin feeding and remain immobile until they emerge as winged adults after the pupal stage.
Video 1. Armor from a false oleander scale, Pseudaulacaspis cockerelli Cooley, being removed to show adult female with eggs residing beneath the armor. Video by Courtney R Buckley, University of Florida.
Female scale insects have three distinct life stages: the egg, nymph, and adult stage. Male citrus snow scales have these stages in addition to a prepupal and pupal stage. The second instar male produces mature armor that protects its bright orange body underneath. The third instar or prepupal stage has distinct eye spots, a more pointed abdomen and faintly visible appendages (Dickens 1968). The fourth instar is referred to as the 'pupal' stage and is quiescent or inactive. During the pupal stage the male develops distinct antennae, legs and a posterior stylet. After the pupal stage, the winged adult male emerges and no longer possesses the waxy covering of the immature stages. The adults do not possess mouthparts and their main goal is to find females and mate.
In comparison, the female adults remain in a larval body form underneath their armor. Even though the females acquire mature sexual organs and can reproduce, their adult larval body form does not have the same characteristics as the adult male, with no antennae, legs or wings (Watson and Berger 1932). Several overlapping generations occur each year in Florida (ScaleNet 2013).
Arias Reverón (1995) found development to be temperature dependent with an optimal range between 25 to 38°C.
Description
Adults
The males and females look considerably different from each other.
Female
The females are 1.5 to 2.25 mm long and have oyster-shell shaped armor with a central longitudinal ridge (Futch, McCoy, and Childers 2012). The shell is brownish-purple to black with a grey border (Fasulo and Brooks 2010). Because of this coloring, the females are difficult to see against tree bark. Beneath the armor, the female body ranges from creamy to bright orange in color (Dickens 1968).

Credit: Lyle Buss, University of Florida

Credit: Ian Stocks, FDACS-Division of Plant Industry
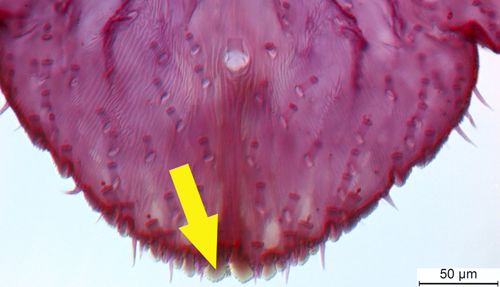
Credit: Ian Stocks, FDACS-Division of Plant Industry
Male
The winged adult male is bright orange in color, has long 10-segmented filiform antennae, four dark purple eye spots, and no mouthparts (Dickens 1968).
Egg
The egg is ovoid, bright orange in color and approximately 0.30 mm in length. Eggs are laid singly underneath the protective covering of the adult female's armor and usually hatch 30 minutes to three hours after being laid. Another egg is not laid until the previous one hatches. Over a two to three month period a female may lay up to 150 eggs.
Nymphs
This life stage begins after the scale insect emerges from the egg. The nymphs are called 'crawlers' because this is the dispersal stage. Nymphs are ovoid, bright yellow in color, with six legs, two five-segmented antennae and two laterally opposed eye spots (Dickens 1968). Crawlers are most abundant in autumn, but may occur throughout the year.
After the first molt, sex differentiation occurs and nymphs begin to form a waxy armor. Males form a white armor and females form a grey armor (Figure 7). The snow white armor color of the immature male is the reason for the species' descriptive name. The armor has three longitudinal ridges; one prominent center ridge, and two marginal ridges (Dickens 1968). The immature male is about 1 mm in length. Females begin producing a white semi-transparent wax covering at the posterior end of the molt's shed exoskeleton (also known as the exuvium).

Credit: S.H. Futch, C.W. McCoy, and C.C. Childers, University of Florida
Hosts
In Florida, the citrus snow scale infests a wide range of citrus species, including:
- Lime (Citrus aurantifolia)
- Sour orange (Citrus aurantium)
- Lemon (Citrus limon)
- Pummelo (Citrus maxima)
- Navel orange (Citrus sinensis)
- Grapefruit (Citrus x paradisi)
In other parts of the world the insect also infests other hosts, including pineapple, soursop, jackfruit, peppers, mandarin, coconut, kumquats, rose mallows, banana, guava, and Spanish moss.
Economic Significance
Commercial citrus production is currently a $9 billion industry and employs thousands of Floridians (Florida Department of Citrus 2013). Florida's 2011–2012 growing season produced ~338 boxes of fruit per acre, with just over 433,000 acres being farmed. Citrus is usually grown for either the fresh market or for juice production, with the fresh fruit returning a larger profit for the grower. The profit for 2011/2012 was ~$4400 per acre for fresh fruit, and ~$3500 per acre for juice production. When the fruit skin is damaged by citrus snow scale, the fruit it not sellable in a fresh market scenario.
The citrus snow scale feeds primarily on the trunk and tree limbs of older citrus trees, feeding on plant juices (Russo and Longo 2004). The scale may also be seen on leaves and fruit if the population is large enough. Early symptoms include reduced tree vigor and fruit production. With prolonged high population densities, the normal growth of the bark is prevented and the toughened bark is not able to expand and may become split or 'hidebound' (Russo and Longo 2004). This split in the bark may allow access for other insect borers and pathogens to invade the damaged tree.

Credit: Michael Rogers, University of Florida

Credit: Michael Rogers, University of Florida
Management
Biological Control
The parasitic wasp Aphytis lingnanensis Compere is effective in helping to control citrus snow scale if it is already established within the grove (Fasulo and Brooks 2010). The introduced ladybird beetle Chilocorus circumdatus Gyllenhal has also shown to be a successful biological control agent (Rogers 2012).
Cultural Control
As the citrus snow scale crawler is the mobile life stage, preventing the distribution of this stage is crucial. Crawlers can be moved by wind, farming equipment and workers in the field. New research has also shown that they may phoretically disperse by attaching themselves to other species, such as pollinating insects (Magsig-Castillo et al. 2010). Farming equipment, such as pruners and fruit shakers, should be cleaned before moving to new groves, and workers should brush their clothing before entering a new area of a grove.
Chemical Control
The effectiveness of biological control agents may be reduced with the application of chemical control agents used in the citrus groves to combat other insect pests. For example, sulfur applications used to control fungal pathogens can adversely affect the parasitic wasp Aphytis lingnanensis (Fasulo and Brooks 2010).
Scouting is an important tool when deciding if an insecticide should be applied. To determine if citrus snow scale crawlers are active, a tree trunk section can be brushed clean with a handheld brush and the same area checked a week later. The need for an application can become evident when high populations of crawlers are seen on patches of bark that had been brushed clean the previous week (Browning et al. 2012).

Credit: Michael Rogers, University of Florida
An insecticide can be applied to manage this pest, but the concentration of the spray and adequate coverage of the tree limbs is important in achieving control. Citrus snow scales will layer themselves on top of each other, seven to eight individuals deep, so a chemical control agent may need to be applied multiple times for the layers to slough off (Fasulo and Brooks 2010).
Florida Citrus Pest Management Guide for Soft-Bodied Insects
Selected References
Anonymous. 2004. Scales. Florida Grower. August: 12–13.
Arias Reverón, JM. 1995. Population studies on the citrus snow scale, Unaspis citri (Comstock). University of Florida PhD Dissertation.
Beardsley Jr JW, Gonzalez RH. 1975. The biology and ecology of armored scales. Annual Review of Entomology 20: 47–73.
Brooks RF, Vitelli MA. 1976. An easily erected tree cage for introducing insect parasites. The Florida Entomologist 59: 1: 67–70.
BugGuide (2003). Citrus snow scale. (19 May 2013)
CABI Compendium, CABI Digital Library (2022). Unaspis citri (citrus snow scale). (7 December 2022)
Culik MP, Martins DS, Ventura JA, Wolff VS. 2008. Diaspididae (Hemiptera: Coccoidea) of Espírito Santo, Brazil. Journal of Insect Science. 8: 17: 1–6.
Dekle GW. 1976. Florida armored scale insects. Arthropods of Florida and neighboring land areas, Volume 3. Florida Department of Agriculture and Consumer Services, Division of Plant Industry. 152.
Dickens TH. 1968. Life history of citrus snow scale Unaspis citri (Comstock). Master of Science Thesis, University of Florida.
Fasulo TR, Brooks RF. (February 2010). Scale pest of Florida citrus. EDIS.
Florida Department of Citrus. 2013. Florida Citrus. (16 July 2013)
Futch SH, McCoy CW, Childers CC. 2012. A guide to scale insect identification. Horticultural Sciences Department, Institute of Food and Agricultural Sciences, University of Florida HS-817.
Hatton Jr TT, Reeder WP. 1963. Effects of the December 1962 freeze on lula and taylor avocado fruits. Proceedings of the Florida State Horticulture Society. 76: 370–374.
Hodges G. 2013. Bureau Chief of Entomology, Nematology and Plant Pathology at Florida Department of Agricultural and Consumer Services, Division of Plant Industry. Personal Interview.
Magsig-Castillo J, Morse JG, Walker GP, Bi JL, Rugman-Jones PF, Stouthamer R. 2010. Phoretic dispersal of armored scale crawlers (Hemiptera: Diaspididae). Journal of Economic Entomology 103: 4: 1172–1179.
Miller DR, Davidson JA. 2005. Armored scale insect pests of trees and shrubs (Hemiptera: Disaspididae). Comstock Publishing Associates. 392–395.
Parsons L. 2007. Freeze forecast. Florida Grower. p. 32.
Robinson FA. 1964. The effects of the December 1962 freeze on citrus honey production in Florida. The Florida Entomologist. 47: 1: 55–56.
Rogers J. 2000. Book Review: A history of Florida citrus freezes. Bulletin of the American Meteorological Society. 81: 3: 590–591.
Rogers M. 2012. Citrus pest spotlight: Citrus snow scale. Citrus Industry May p. 12.
Russo A, Longo S. 2004. Diagnostic protocols for regulated pests: Unaspis citri. European and Mediterranean Plant Protection Organization. Bulletin 34: 299–301.
ScaleNet: A database of the scale insects of the world. (June 2017). Unaspis citri (Comstock). (29 June 2017)
Soares AO, Elias RB, Schanderl H. 1997. Encarsia citrina (Crawford) (Hymenoptera, Aphelinidae), a parasitoid of Unaspis citri (Comstock) and Lepidosaphes beckii (Newman) (Homoptera, Diaspididae) in citrus orchards of São Miguel island (Azores). Bol. San. Veg. Plagas. 23: 449–456.
Stansley PA, Rogers ME. December 1995. Revised September 2013 and April 2016. 2016 Florida Citrus Pest Management Guide: Ch. 11 Soft-Bodied Insects Attacking Foliage and Fruit. EDIS. (29 June 2017)
Stocks I. 2013. Biologist Scientist IV, Florida Department of Agricultural and Consumer Services, Division of Plant Industry. Personal Interview.
Stout, RG. 1964. Florida citrus fruit and tree losses from the December 1962 freeze. Department of Agricultural Economics, University of Florida. Agricultural Economics Mimeo Report EC 64–7.
Terry LI, Edwards GJ. 1989. Efficacy of densitometric and multispectral techniques for monitoring infestations of citrus snow scale on citrus bark. Journal of Photogrammetric Engineering and Remote Sensing. 55: 10: 1471–1475.
United States Department of Agriculture, National Agricultural Statistics Service. (September 2012). Citrus fruits 2012 summary.
Watson JR, Berger EW. June 1932. Citrus insects and their control. Agricultural Extension Service, University of Florida. Bulletin 67.