The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction

Credit: Justin Bredlau, Virginia Commonwealth University
Cotesia congregata (Say) is a gregarious endoparasitoid that attacks more than a dozen species of sphingid caterpillars and a few known semi-permissive noctuid hosts. Semi-permissive hosts are not fully susceptible to the immunosuppressant polydnavirus associated with Cotesia congregata. Thus, they exhibit an immune response to some of the parasitoid eggs that are oviposited into the hemocoel, and may not always become parasitoid hosts. Its most notable host, especially in the southeastern US, is the tobacco hornworm, Manduca sexta (Linnaeus). This species of Cotesia has been widely used as a model system in insect physiology. It has also been used to examine insect learning in host-parasitoid-plant interactions.
Synonymy
Apanteles (Protapanteles) augustus Viereck, 1917
Apanteles congregatus (Say, 1836)
Cotesia augusta (Viereck, 1917)
Cotesia utilis (French, 1880)
Microgaster congregatus Say, 1836
Microgaster utilis French, 1880
Cotesia congregata Mason 1981
Currently, there are around 400 described species of the genus Cotesia (Shaw and Huddleston 1991). It is estimated that the entire genus comprises 1500-2000 species worldwide (Mason 1981).
Distribution
Cotesia congregata is widely distributed throughout North America, Central America, South America, and the West Indies (Krombein 1979).
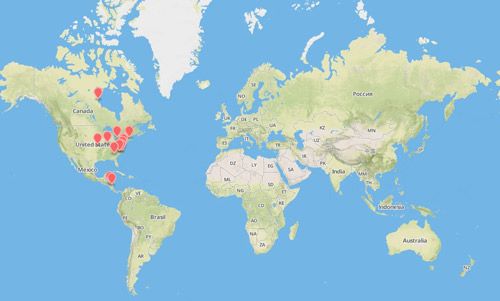
Credit: Map created using www.mapbox.com.
Description and Life Cycle
Eggs
The eggs are white and wedge-shaped, measuring 0.12–0.16 mm in length and approximately 0.04 mm in diameter towards the larger end (Fulton 1940). During a single oviposition, around 65 eggs are oviposited into the hemolymph of the larval host (range: 5–162) (Lawrence 2010). It has been shown that some larvae can emerge from the egg into the hemolymph in as little as two to three days after oviposition at 29°–30°C (Fulton 1940) (Figure 3).
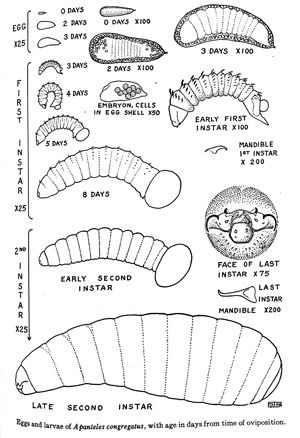
Credit: Adapted from Fulton, 1940.
Larva
Larvae are pale yellow-white and grub-like in appearance. The first instar larvae are free moving in the body cavity close to the epidermal layer of the cuticle. They are around 0.4–0.5 mm in size at the time of hatching, and will continue to grow up to 2.4 mm in length by the end of the first instar (8 days). The first instar larvae also have a short caudal appendage (tail), small curved mandibles (0.04 mm), and eleven body segments each with a row of curved bristles. These features become less apparent from the early first instar to the late first instar. During the second instar, the larvae will grow from around 2.4 mm to 4.5–5.0 mm in length. The larvae will lose the curved bristles that were found in the previous instar and gain an additional posterior body segment. It is at this stage that the larva will begin to tear through the host's cuticle, using its mandibles in a back and forth motion, and emerge to continue its development.
Emergence of wasp larvae from the host cuticle is largely synchronous and subsequent emergence of wasp larvae is rare. The number of wasp larvae emerging from the host can vary from a few to well over 300 depending on the number of females that oviposit into the larval host (Fulton 1940). In total, the time from oviposition to emergence from the host cuticle is around 12–16 days, though the fastest time observed from oviposition to emergence was only five days (de Buron and Beckage 1992; Gilmore 1938). Upon emergence, the wasp larvae will undergo another larval molt (3rd instar) before spinning conspicuous white cocoons where they will develop into pupae and adults.
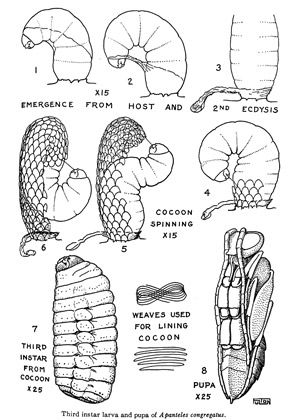
Credit: Adapted from Fulton 1940.
Pupa
The pupae are formed after cocoon construction is completed. The period spent in the cocoon can range from 3–8 days depending on the temperature (de Buron and Beckage 1992; Fulton 1940; Gilmore 1938). Larvae that build their cocoons in late winter will overwinter as larvae and pupate in the spring of the following year (Fulton 1940). The cocoons will stay on the host for a while, but will eventually fall down to the soil.
Adult
Body length is around 2–3 mm for both adult males and females with antennae length nearly equal to body length. The body and eyes are black and the wings are translucent, with an opaque dark spot on the outer edge of each wing called the pterostigma. The shield-shaped plates where the wings meet the body (also called the tegulae) are pale yellow. The ovipositor of female Cotesia congregata is about 0.5 mm long and tapers from a wide base to a very narrow middle and tip that are uniform in thickness.

Credit: Justin Bredlau, Virginia Commonwealth University
Behavior
Females of Cotesia congregata display both inherent and learned responses to chemical cues that are released from plant leaves during the feeding of their caterpillar hosts (Kester and Barbosa, 1994; Turlings et al. 1990; Lentz-Ronning and Kester 2013). These cues allow the females to locate adequate hosts due to the high level of specificity between the chemicals released by the plants and the host species. Upon recognition of the chemical cues, females will fly, upwind, to the location of the host plant. Once on the host plant, females will engage in a host searching behavior which involves moving their antennae back and forth rapidly to further sense additional host cues such as cuticular semiochemicals (Kester and Barbosa 1991). Once the female has located a suitable host she will insert her ovipositor through the cuticle into the hemocoel and deposit her eggs inside of the caterpillar. Wasp larvae develop inside of the caterpillar host, feeding on the hemolymph, and eventually emerge to spin small white cocoons on the cuticle of the host. Larvae complete their development into adults and emerge from the cocoons to mate and find new hosts.
Adult males of Cotesia congregata engage in courtship behavior prior to mating (Bredlau et al. 2013). Males attract females with an auditory display that is produced by the wings beating rapidly, and is characterized by a buzz and a series of distinct high amplitude 'boing' noises. Multiple males will perform this courtship ritual in the presence of a single female wasp.

Credit: Justin Bredlau, Virginia Commonwealth University
Viral Symbiosis
When female Cotesia congregata oviposit into the hemocoel of their larval host they simultaneously inject venom and a symbiotic virus called a polydnavirus (one of the bracoviruses) into the hemocoel. The injected virus prevents the host's immune system from attacking and killing the wasp eggs through encapsulation. The symbiotic polydnavirus also prevents the formation of host storage proteins, leaving more available resources in the hemolymph for the wasp larvae as they develop (Espagne 2004). The injected venom and special hormone-releasing cells from the wasp eggs (teratocytes) work in conjunction with the polydnavirus to arrest the development of the larval host (Beckage 1994).

Credit: Justin Bredlau, Virginia Commonwealth University
Hosts
Cotesia congregata attacks members of the family Sphingidae (the sphinx moths), including but not limited to the following species (Sharkey et al. 2005; Fulton 1940; Molina-Ochoa et al. 2003):
Ceratomia catalpae (Boisduval) (the catalpa sphinx)
Darapsa myron (Cramer) (the Virginia creeper sphinx)
Darapsa versicolor (Harris) (the hydrangea sphinx)
Dolba hyloeus (Drury) (the paw paw sphinx)
Eumorpha achemon (Drury) (the achemon sphinx)
Eumorpha pandorus (Hübner) (the Pandora sphinx)
Hemaris diffinis (Boisduval) (the snowberry clearwing)
Lapara bombycoides Walker (the northern pine spnix)
Manduca quinquemaculata (Haworth) (the five-spotted hawkmoth)
Manduca sexta (Linnaeus) (the tobacco hornworm)
Paratrea plebeja (Fabricius) (the plebeian sphinx)
Sphecodina abbottii (Swainson) (Abbott's sphinx)
Sphinx chersis (Hübner) (the great ash sphinx or the northern ash sphinx)
Sphinx kalmiae Smith (the laurel sphinx)
Spodoptera frugiperda (Smith)(fall armyworm)
Trichoplusia ni (Hübner) (the cabbage looper) (semi-permissive host)
Importance
Cotesia congregata is an important natural enemy of the tobacco hornworm, Manduca sexta, a detrimental pest species that feeds on many plants in the Solanaceae (tobacco, pepper, tomato, etc.) family.
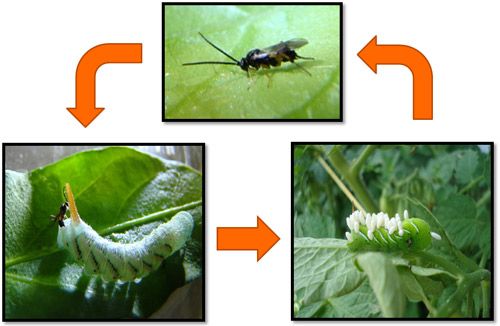
Credit: Justin Bredlau, Virginia Commonwealth University
Selected References
Adamo SA. 2005. Parasitic suppression of feeding in the tobacco hornworm, Manduca sexta: Parallels with feeding depression after an immune challenge. Archives of Insect Biochemistry and Physiology 60: 185–197.
Beckage NE, Tan FF, Schleifer KW, Lane RD, Cherubin LL. 1994. Characterization and biological effects of Cotesia congregata polydnavirus on host larvae of the tobacco hornworm, Manduca sexta. Archives of Insect Biochemistry and Physiology 26: 165–195.
Bredlau JP, Mohajer YJ, Cameron TM, Kester KM, Fine ML. 2013. Characterization and Generation of Male Courtship Song in Cotesia congregata (Hymenoptera: Braconidae). PLoS ONE 8: e62051.
de Buron I, Beckage NE. 1992. Characterization of polydnavirus (PDV) and virus-like filamentous particle (VLFP) in the braconid wasp Cotesia congregata (Hymenoptera: Braconidae). Journal of Invertebrate Pathology 59: 315–327.
Espagne E. 2004. Genome sequence of a polydnavirus: insights into symbiotic virus evolution. Science 306: 286–289.
Fulton BB. 1940. The hornworm parasite, Apanteles congregatus (Say) and the hyperparasite, Hypopteromalus tabacum (Fitch). Annals of the Entomological Society of America 33: 231–244.
Gilmore JU. 1938. Notes on Apanteles congregatus (Say) as a parasite of tobacco hornworms. Journal of Economic Entomology 31: 712–715.
Illinois Natural History Survey: Illinois Natural History Survey (accessed through GBIF data portal, http://data.gbif.org/datasets/resource/13517, 2014-05-08).
iNaturalist.org: iNaturalist research-grade observations (accessed through GBIF data portal, http://data.gbif.org/datasets/resource/14026, 2014-05-08).
Kester KM, Barbosa P. 1991. Post-emergence learning in the insect parasitoid, Cotesia congregata (Say) (Hymenoptera: Braconidae). Journal of Insect Behavior 4: 727–742.
Kester KM, Barbosa P. 1994. Behavioral responses to host foodplants of two populations of the insect parasitoid Cotesia congregata (Say). Oecologia 99: 151–157.
Krombein KV. 1979. Catalog of Hymenoptera in America north of Mexico. Smithsonian Institution Press, Washington D.C., US.
Lawrence SE. 2010. Feeding behaviors of larval Manduca sexta parasitized by Cotesia congregata. (Order No. 1487815, State University of New York at Binghamton). ProQuest Dissertations and Theses, 56. Retrieved from http://search.proquest.com/docview/847404852?accountid=10920. (847404852).
Lentz-Ronning AJ, Kester KM. 2012. Effect of sequential learning experiences on searching responses and sex ratio allocations of the gregarious insect parasitoid, Cotesia congregata (Say) (Hymenoptera: Braconidae). Journal of Insect Behavior 26: 165–175.
Mason WRM. 1981. The polyphyletic nature of Apanteles förster (Hymenoptera: Braconidae): a phylogeny and reclassification of Microgastrinae. Memoirs of the Entomological Society of Canada 115: 1–147.
Molina-Ochoa J, Carpenter JE, Heinrichs EA, Foster JE. 2003. Parasitoids and parasites of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean Basin: an inventory. Florida Entomologist 86: 254–289.
Ratnasingham S, Hebert PDN. 2007. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Molecular Ecology Notes 7, 355–364. (http://www.boldsystems.org/index.php/TaxBrowser_Taxonpage?taxid=106531)
Sharkey MJ, Whitfield J, Seltmann K. 2005. Species home pages for economically important species parasitic wasps of the genus Cotesia (Hymenoptera: Braconidae). Both at http://www.uky.edu/~mjshar0/genera/Cotesia/congregata.html
Shaw MR, Huddleston T. 1991. Classification and biology of braconid wasps (Hymenoptera: Braconidae). Handbooks for the Identification of British Insects (ed. by W. R. Dolling and R. R. Askew), Vol. 7, pp. 1–126. Royal Entomological Society of London, London.
Thorpe KW, Barbosa P. 1986. Effects of consumption of high and low nicotine tobacco by Manduca sexta (Lepidoptera: Sphingidae) on survival of gregarious endoparasitoid Cotesia congregata (Hymenoptera: Braconidae). Journal of Chemical Ecology 12: 1329–1337.
Turlings TCJ, Tumlinson JH, Lewis WJ. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250: 1251–1253.
Witting-Bissinger BE, Orr DB, Linker HM. 2008. Effects of floral resources on fitness of the parasitoids Trichogramma exiguum (Hymenoptera: Trichogrammatidae) and Cotesia congregata (Hymenoptera: Braconidae). Biological Control 47: 180–186.