Introduction
Coconut scale, Aspidiotus destructor Signoret (Hemiptera: Diaspididae), is a pest in over 60 plant families and is found globally in tropical and subtropical areas (Davidson and Miller 1990; Ben-Dov 2014). This armored scale was described by Signoret in 1869 and appears to be native to South Asia but has spread around the world, mainly on infested coconut and banana (UK CAB International 1966). The insect feeds on plant sap from leaves, stems and fruits, causing yellowing, tissue distortion and die back. The coconut scale is a pest of concern on coconut and other perennial crops due to its relatively short life cycle of around 35 days and multiple overlapping generations per year. Coconut scale is known to be dispersed by birds, bats and insects as well as wind (Taylor 1935).

Credit: Salahud din, University of Florida
Synonymy
Other scientific names:
- Aspidiotus cocotis Newstead (1893)
- Aspidiotus lataniae Green (1896)
- Aspidiotus transparens Green (1890)
- Aspidiotus translucens Cockerell and Robinson (1915)
- Aspidiotus watanabei Takagi (1969)
- Temnaspidiotus destructor (Signoret) Borchsenius
Other common names:
- bourbon scale
- bourbon aspidiotus
- transparent scale
Distribution
Coconut scale has been recorded worldwide in tropical and subtropical areas, including China, Southeast Asia, India, Pakistan, Russia, Brazil, Central America, and Caribbean, the Pacific Islands, Africa and North America (UK CAB International 1966; Bennett and Alam 1985; Tao 1999; Claps et al. 2001). Coconut scale is present in nearly all coconut growing countries of the world. In the United States, coconut scale has been recorded in Connecticut, Florida, Georgia, Hawaii, Pennsylvania, and Puerto Rico (Nakahara 1982; Heu 2002). In more northern regions, it is found only in greenhouses or other enclosed areas (Danzig and Pellizzari 1998).

Credit: UK CAB International 1966, Bennett and Alam 1985, Tao 1999, Claps et al. 2001
Description
Coconut scale resembles other armored scales in that the body is protected by a waxy cover. Infestations may be noted by the formation of closely packed colonies composed of what resemble miniature fried eggs. Females develop through two nymphal stages, while males have an additional non-feeding pre-pupal stage (four immature stages). Positive identification of coconut scales requires that adult females be slide mounted and the diagnostic characters observed at high magnification.
Eggs
Freshly laid eggs are smooth, elongate and whitish, becoming pale yellow over time. Eggs are laid under the scale cover around the body of the female. Mean length and width of eggs are 0.22 mm (0.009 in) and 0.09 mm (0.004 in), respectively (Salahud din et al. 2015).
First Instar Nymphs
Newly hatched nymphs (also called crawlers) are free-moving and recognized by the presence of legs, antennae, and a pair of bristles at the tip of abdomen. Crawlers are light green to yellowish brown, translucent and somewhat oblong, with an average length and width of 0.23 mm (0.009 in) and 0.11 mm (0.004 in), respectively (Salahud din et al. 2015).
Second Instar Female
Females remain pale yellow, circular and somewhat transparent in the second instar, which lasts for 8–10 days. Female nymphs are 0.6–1.1 mm (0.02–0.04 in) long (Williams and Watson 1988).
Male Nymphs
Development of male characteristics starts in the middle of the second instar. Male covers become reddish brown and more elliptical in shape, and then transform in stages into the pre-pupal, pupal and adult stages. The second instar lasts for 5–8 days for males (Williams and Watson 1988). The male pre-pupal and pupal stages are spent under the scale produced by the second instar.
Adult Females
Adult female coconut scales have a circular or broadly oval cover that is 1.5–2.0 mm (~1/16 in) in diameter. The cover is flat and translucent with a subcentral pale exuviae. Bodies of slide-mounted specimens are 0.7–1.2 mm (0.03–0.05 in) in length with a pyriform and membranous body. Diagnostic characteristics include three pairs of lobes with no indication of a fourth pair; plates long, flat and fringed; and with sclerotization on the dorsum of the pygidium (Williams and Watson 1988).
Adult Males
Adult male coconut scales are small, two-winged, reddish, gnat-like insects with eyes, antennae, three pairs of legs and long appendages. Adult males do not feed and are short lived.

Credit: Salahud din, University of Florida
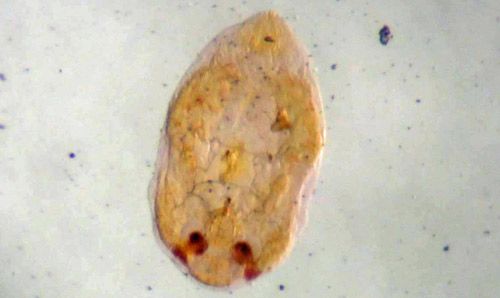
Credit: Salahud din, University of Florida

Credit: Salahud din, University of Florida

Credit: Salahud din, University of Florida
Life Cycle
Adult females lay 28–65 eggs in concentric circles under the scale cover over a period of 11–13 days and may produce 3 or 4 consecutive batches in their lifetime (Taylor 1935). Crawlers may emerge while the female has already started laying the next batch of eggs (personal observation). The crawlers move over the leaf surface for 2–48 hours to find a feeding site (Taylor 1935; Waterhouse and Norris 1987). After settling, females remain sessile throughout their development; adult males undergo a pseudo-pupation, develop a pair of wings and can disperse by flying to find mates (Ghauri 1962). Females release pheromones through their anus to attract males (Moreno 1972). Sexual reproduction is influenced by food availability, but typically the sex ratio is 1:1 (male: female); although both male and female colonies have been observed (Ahmad and Ghani 1972; Taylor 1935).
Crawlers are the primary dispersal stage of coconut scale within and between host trees. Coconut scale development depends upon climatic conditions: at a mean temperature of 30°C (86°F) and 65% relative humidity, egg to adult development was 35.5 days for females and 28.5 days for males, with 3 or 4 generations from July to December on mango plants in Pakistan (Salahud din et al. 2015).
Hosts
Ben-Dov (2014) reported several hundred hosts from over 60 plant families. Common perennial hosts include tropical fruits and ornamental plants, including banana, coconut, camellia, guava, mango, palm, papaya, breadfruit, ginger, bird of paradise, sugarcane, ficus, apple, plumeria, avocado, citrus, grape, and palms.
Economic Importance
Coconut scale is considered a major threat to coconut palms throughout the world. It infests at high densities on the undersurface of coconut leaves, as well as on the frond stalks, flower clusters and young fruit. Coconut scale was accidentally introduced into the Pacific Islands and became a serious pest until the introduction of parasitoids and predators which achieved significant suppression (Waterhouse and Norris 1987). It is also an economic pest of mango in Asia, Africa, and South America (Chua and Wood 1990; Kumari et al. 2014).
This species is reported to preferentially infest older leaves of mango, but it also causes blemishes on the skin of mango fruits which reduces their value. Coconut scale infests fruits of oil palms and banana and reduces fruit quality (Chua and Wood 1990). Coconut scale was first reported in Hawaii in 1968 on papaya, banana, and apple; and remains a pest of concern in that region (Kessing and Mau 2007). Removal of sap from leaves, petioles, peduncles, and fruits leads to discoloration, depressions, and tissue distortions on leaves. Coconut scales may possibly introduce toxins into the plant through their saliva (Waterhouse and Norris 1987).

Credit: Salahud din, University of Florida

Credit: Salahud din, University of Florida

Credit: Salahud din, University of Florida

Credit: Salahud din, University of Florida
Management
Biological Control
Over 40 species of parasitoids and predators, and several fungal pathogens, are known to attack coconut scale (Ben-Dov et al. 2014). In the absence of these natural enemies, population explosions of this pest can occur. Aphytis melinus DeBach and Aphytis lingnanensis Compere (Hymenoptera: Aphelinidae) are the most common parasitoid species controlling the coconut scale populations in Fiji and the Philippines (DeBach 1974; Watson et al. 2015). Aphytis melinus and/or another parasitoid, Comperiella bifasciata Howard (Hymenoptera: Encyrtidae), were introduced into Hawaii, California, Argentina, and other regions for the control of coconut scale. In Pakistan, Pakencyyrtus pakistanensis Ahmad (Hymenoptera: Encyrtidae) was reported as an important parasitoid in mango (Ahamad and Ghani, 1972). Coccinellid ladybird beetle predators of coconut scale include Chilocorus spp., Telsimia nitida Chapin, Pseudoscymnus anomalus Chapin, and Cryptognatha spp.

Credit: Jack Kelly Clark, used with permission from University of California Statewide IPM Program
Cultural Control
Pruning and training of fruit trees and proper disposal of infested leaves, branches and twigs will help control scale insects on nursery plants and trees. Excessive use of plant fertilizers contributes to scale outbreaks.
Chemical Control
Various insecticides are registered for control of armored scales in ornamental and fruit crops. Crawler stages are generally the most susceptible to insecticides. Contact action insecticides, including horticultural oils, become progressively less effective once the scale insects develop their waxy cover. Insect growth regulators may be effective, provided they are applied when the immature stages are present. Location of the insect on the plant, growth stage of the plant, and solubility of the insecticide influence the effectiveness of systemic insecticides applied for control of armored scales. Several spray applications at 15–20 day intervals may be necessary for complete management of a heavy infestation. The toxicity of insecticides to parasitoids and other beneficial insects should be considered before starting a spray program for scale insects. Since coconut scale is a quarantine pest in many areas, phytosanitary treatment with gamma irradiation has been developed as a potential control measure for this scale (Follet 2006).
Selected References
Ahmad R, Ghani MA. 1972. Studies on Aspidiotus destructor Sign. (Hemiptera: Diaspididae) and its parasites, Aphytus melinus Debach (Hymenoptera: Aphelinidae) and Pakencyyrtus pakistanensis Ahmad (Hymenoptera: Encyrtidae) in Pakistan. Commonwealth Institute of Biological Control Technical Bulletin 15: 51–57.
Ben-Dov Y, Miller DR, Gibson GAP. 2014. ScaleNet. (15 June 2022)
Bennett FD, Alam MM. 1985. An annotated check-list of the insects and allied terrestrial arthropods of Barbados. Bridgetown, Barbados; Caribbean Agricultural Research and Development Institute, pp. 6–81.
Chua TH, Wood BJ. 1990. Other tropical fruit trees and shrubs. In D. Rosen (ed.), armored scale insects, their biology, natural enemies and control. World Crop Pests. Elsevier, Amsterdam, the Netherlands, 4: pp. 543–552.
Claps LE, Wolff VRS, González RH. 2001. Catálogo de las Diaspididae (Hemiptera: Coccoidea) exóticas de la Argentina, Brasil y Chile. Revista de la Socieded Entomológica Argentina 60: 9–34.
Danzig EM, Pellizzari G. 1998. Diaspididae. In Kozár F, (Ed.), Catalogue of Palearctic Coccoidea. Hungarian Academy of Sciences. Budapest, Hungary: pp. 172–370.
Davidson JA, Miller DR. 1990. Ornamental plants. In Rosen, D (ed.) Armored scale insects, their biology, natural enemies and control. Elsevier, Amsterdam, the Netherlands, 4 (B): pp. 603–632.
DeBach P. 1974. Coconut scale in Fiji. In Biological Control by Natural Enemies. DeBach P (ed.). Cambridge Univ. Press, Cambridge UK, pp. 133–135.
Follet PA. 2006. Irradiation as a phytosanitary treatment for Aspidiotus destructor (Homoptera: Diaspididae). Journal of Economic Entomology 99: 1138–1142.
Ghauri M. 1962. The morphology and taxonomy of male scale insects (Hemiptera: Coccoidea). British Museum (Natural History). Adlard and Son, Dorking, UK. 221 pp.
Heu RA. 2002. Distribution and host records of agricultural pests and other organisms in Hawaii. USA: State of Hawaii Department of Agriculture.
Kessing JLM, Mau RFL. 2007. Aspidiotus destructor (Signoret). Crop Knowledge Master, University of Hawaii, http://www.extento.hawaii.edu/kbase/crop/type/a_destru.htm (12 February 2015)
Kumari DA, Anitha V, Lakshmi BKM. 2014. Evaluation of insecticides for the management of scale insect in mango (Mangifera indica). International Journal of Plant Protection 7: 64–66.
Moreno DS. 1972. Location of the site of production of the sex pheromone in the yellow scale and the California red scale. Annals of the Entomological Society of America. 65: 1283–1286.
Nakahara S. 1982. Checklist of the Armored Scales (Homoptera: Diapididae) of the Conterminous United States. Washington, USA: USDA, Animal and Plant Health Inspection Service, Plant Protection and Quarantine, 110 pp.
Salahud din, Rahman HU, Khan I, Daud MK. 2015. Biology of coconut scale, Aspidiotus destructor Signoret (Hemiptera: Diaspididae), on mango plants (Mangifera sp.) under laboratory and greenhouse conditions. Pakistan Journal of Zoology.
Tao C. 1999. List of Coccoidea (Homoptera) of China. Taichung, Taiwan: Taiwan Agricultural Research Institute, Wufeng, 1–176.
Taylor THC. 1935. The Campaign against Aspidiotus destructor, Signoret in Fiji. Bulletin of Entomological Research 26: 1–102.
UK CAB International, 1966. Distribution maps of plant pests, June. Wallingford, UK: CAB International, Map 218. (10 February 2015)
Waterhouse DF, Norris KR. 1987. Aspidiotus destructor Signoret in Biological Control Pacific prospects. Inkata Press, Melbourne. Chapter 8: 454 p.
Watson GW, Adalla CB, Shepard BM, Carner GR. 2015. Aspidiotus rigidus Reyne (Hemiptera: Diaspididae): a devastating pest of coconut in the Philippines. Agricultural and Forest Entomology 17: 1–8.
Williams DJ, Watson GW. 1988. The scale insects of the tropical South Pacific region. Part 1, the armored scales (Diaspididae). CAB International, Wallingford, UK. 290 p.