The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Psorophora ferox, (Figures 1 and 2) known unofficially as the white-footed woods mosquito (King et al. 1942), is a mosquito species native to most of North and South America. It is a multivoltine species, having multiple generations each year. The mosquito is typical of woodland environments with pools that intermittently fill with rain or flood water. Several viruses have been isolated from the mosquito, but it is generally not thought to play a major role in pathogen transmission to humans. However, the mosquito is known to frequently and voraciously bite people.

Credit: Chris Holderman, UF/IFAS

Credit: Chris Holderman, UF/IFAS
Synonymy
Culex posticatus Wiedemann (1821)
Culex musicus Say (1829)
Janthinosoma echinata Grabham (1906)
Janthinosoma sayi Dyar and Knab (1906)
Janthinosoma terminalis Coquillett (1906)
Janthinosoma vanhalli Dyar and Knab (1906)
Janthinosoma coquillettii Theobald (1907)
Janthinosoma sayi Theobald (1907)
Janthinosoma jamaicensis Theobald (1907)
Aedes pazosi Pazos (1908)
Janthinosoma centrale Brethes (1910)
Compiled by WRBU (2013b)
Distribution
Psorophora ferox is widely distributed, occurring in numerous localities ranging from southeastern Canada (Ontario) in the north, through the eastern US (Figure 3), Mexico, and Central America, the Caribbean, to South America (Carpenter and LaCasse 1955; Darsie and Ward 2005). Countries where the mosquito has been collected include Argentina, the Bahamas, Belize, Bolivia, Brazil, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Nicaragua, Panama, Peru, Saint Lucia, Suriname, Uruguay, and Venezuela (compiled by WRBU 2013b).
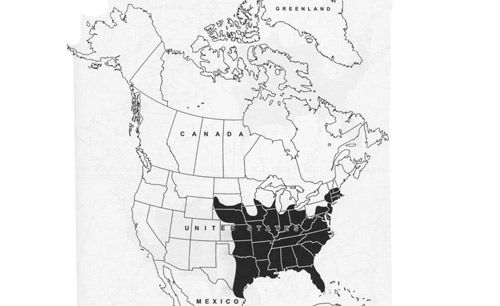
Credit: Darsie and Ward RA (2005)
Description and Life Cycle
Eggs
Psorophora ferox eggs from a Florida population ranged in length from 845 to 963 µm (0.033 to 0.038 inches) and width from 227 to 265 µm (0.0089 to 0.0104 inches) (Linley and Chadee 1990). An individual female was shown to oviposit approximately 80 eggs in one gonotrophic cycle (one blood feeding event) (Micieli 2003). The eggs are placed in or near temporary pools, and may diapause and resist desiccation until rain or flooding inundates the eggs with water, thereby stimulating the eggs to hatch into larvae (Micieli 2003). Alternatively, the eggs may not diapause and hatch shortly after being deposited by the female.
Larvae
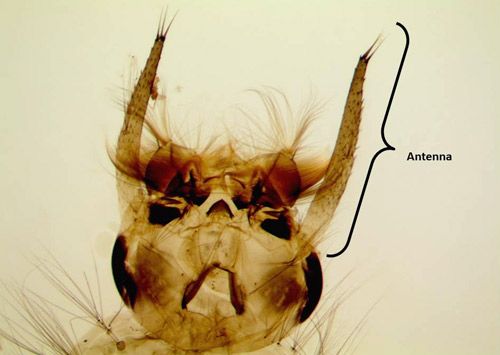
Larvae are aquatic and were reported to be almost 8 mm (0.3125 in) long in collections of last instar larvae from Jamaica (Grabham 1903) (Figure 4). Development is relatively short and takes 5–7 days (Micieli 2003). In Florida larvae are reported to live in well-shaded pools or depressions in palm and oak woodlands. Larvae can be identified to genus by a well-developed ventral brush (seta 4-X) (Figure 5), a siphon with 3–5 widely spaced pectin spines on the basal fourth (Figure 5), with one pair of setae on the siphon, and a complete saddle pierced midventrally by a row of precratal setae (Darsie and Ward 2005; WRBU 2013a). Psorophora ferox have antennae as long or longer than the head capsule (Figure 6), seven or more precratal setae and Setae 6-IV-VI single or double branched (Darsie and Ward 2005; WRBU 2013a). As with all mosquitoes, larvae molt through four instars (stages) before molting into the pupal stage.

Credit: Chris Holderman, UF/IFAS
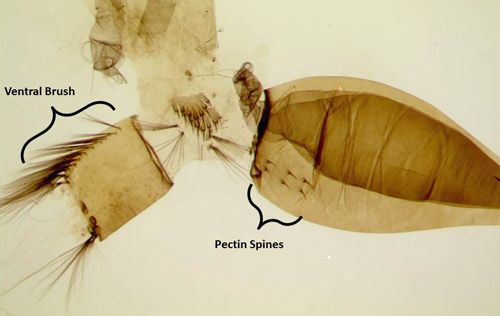
Credit: Chris Holderman, UF/IFAS
Credit: Chris Holderman, UF/IFAS
Pupae
Like mosquito larvae, mosquito pupae are aquatic. The pupae possess a pair of respiratory trumpets, attached to the thorax, which allow gas exchange (Gillett 1972). Similar to other mosquito pupae, the pupae of Psorophora ferox are shaped like a comma and do not feed. An individual mosquito will typically remain a pupa for 1–2 days, then molt into an adult.
Adults
In North America, adults in the genus Psorophora are identified by having a pointed abdomen and possessing both pre- and post-spiracular setae. Psorophora feroxis characterized by dark wing scales (Figures 8 and 9), hind tarsomeres with a shaggy appearance and tarsal sub-segments 5, 4, and often 3 white (Figure 10), and a scutum with both dark brown and golden-yellow scales (Figure 11) (Darsie and Ward 2005; WRBU 2013a). The common name originates from adult habitat (woodlands) and the characteristic white tarsomeres on the hind legs.

Credit: Chris Holderman, UF/IFAS

Credit: Chris Holderman, UF/IFAS

Credit: Chris Holderman, UF/IFAS

Credit: Chris Holderman, UF/IFAS
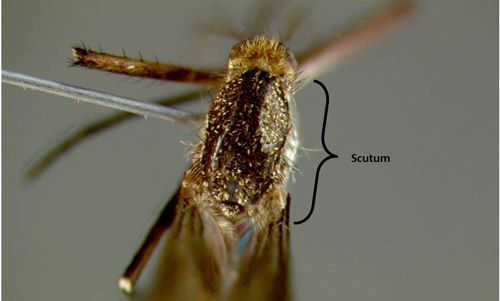
Credit: Chris Holderman, UF/IFAS
After eclosing from the pupa, adults will rest near the ground or on the water's surface (Nielsen 1964). After resting for several hours the adults will disperse into the forest (Nielsen 1964). Female adults will frequently bite people and other animals (Micieli 2003) and have been reported to be crepuscular (feeding at sunrise and sunset), and diurnal (feeding during the daytime) with possible feeding occurring during the night (Arnett 1949). Females have been shown to mostly feed on mammal hosts, but may feed on birds (Molaei et al. 2008). Adults are thought to have a lifespan of 4 to 9 days in the wild (Nielsen 1964).
Males have antennal fibrillae, sensory organs used to detect the wing beats of female mosquitoes. In Psorophora ferox, the fibrillae were observed to be influenced by temperature: permanently extended in warmer periods, but only extended during swarming in colder periods (Nielsen 1964). Nielsen (1964) reported that adults swarm during the day in open areas of the forest when it is cloudy or overcast; during these swarms females seek males in order to mate with them. Daytime swarms were reported to be 1 to 2 meters in diameter or less and located in shaded open areas under vegetation (Nielsen 1964). Wind, birds, other insects, and people have been reported to initiate mating swarms (Nielsen 1964). At dusk, the swarms would move upward under large trees and would cease soon after sunset. Swarms have occasionally been noted shortly after daybreak. An individual usually did not swarm longer than a few minutes at a time (Nielsen 1964).
Medical and Veterinary Importance
Female Psorophora ferox are reported to be persistent and painful biters when host seeking, and frequently bite people (Magnarellli 1980). Numerous viruses have been isolated from Psorophora ferox. However, the sylvatic (affecting wild rather than domestic animals) nature of this mosquito likely prevents it from being a major vector in transmitting pathogens to people.
The mosquito has been shown to transmit Venezuelan equine encephalomyelitis virus (VEE) in laboratory experiments (Chamberlain et al. 1956). VEE primarily infects horses and other equids; although, it may infect humans. A vaccine is available for horses (Weaver et al. 2004). Human infection typically only occurs with viral amplification in horses in epizootic conditions. Human cases in Central and South America have been reported (Weaver et al. 2004).
Psorophora ferox was a suspected vector of Rocio virus because one mosquito pool (subsample at a field site) was found to contain the virus (Lopes et al. 1981). However, later evaluations demonstrated that Psorophora ferox, from a colony that originated in Louisiana, was an inefficient vector (Mitchell et al. 1981). Rocio virus causes viral encephalitis and was isolated in an epidemic in southern Brazilian forests (Coimbra et al. 2008). The virus has not been isolated elsewhere. There remains little understanding of the vector-pathogen relationship with Rocio virus in the field.
In a survey conducted during the 1999–2000 West Nile virus epidemic in New York, one pool of Psorophora ferox was reported to contain West Nile virus (Kulasekera et al. 2001). Comparatively, this was a low value and the major vectors in the area were thought to be other mosquito species (Kulasekera et al. 2001). In follow up surveillance, the role of Psorophora ferox in the maintenance or transmission cycle of West Nile virus was thought to be minor (Andreadis et al. 2004).
Turell et al. (2005) reported isolations of several viruses from Psorophora ferox collected in the Amazon basin including: Ilheus virus, Una virus, and an unidentified Bunyamwera-group virus. Ilheus virus can cause encephalitis-like symptoms. It typically is transmitted between mosquitoes and birds, can be sporadically isolated from humans, and has been isolated only from Central and South America and Trinidad (Johnson et al. 2007). Una virus is widely distributed in tropical and subtropical regions of Central and South America and has been isolated from humans, yet is not associated with any human disease (Diaz et al. 2003).
Dermatobia hominis, the human bot fly, does not lay its eggs on the larval host directly, but instead lays its eggs on blood-feeding flies as a part of its normal developmental cycle. The bot fly has been reported to frequently lay eggs on the abdomen of female Psorophora ferox (Arnett 1949). After egg transfer, the mosquito locates and feeds upon a mammalian host. Proximity to the host stimulates the bot eggs to hatch and subsequently enter the skin where they complete development. Dermatobia hominis only occurs in parts of Mexico, south through South America, therefore infestation by this fly only poses a risk to people in the US that travel to these areas. Such visitors may bring back bots after a bite by a mosquito harboring bot fly eggs, but local transmission in the US is quite rare.
Management
Mosquito reduction programs do not typically target Psorophora ferox because they are a woodland floodwater species not often encountered by the general populace. To reduce exposure to biting insects, avoiding outdoor activities during the crepuscular (dawn and dusk) periods, or when most biting insects are active, and wearing long sleeves and pants is recommended. Use of Environmental Protection Agency (EPA) registered mosquito repellents is recommended by the Centers for Disease Control and Prevention (CDC) when fishing, hunting, and engaging in other outdoor activities that may bring people into contact with mosquitoes or other biting arthropods. For more information on specific repellents consult the CDC's website http://www.cdc.gov.
Selected References
Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. 2004. "Epidemiology of West Nile virus in Connecticut: A five-year analysis of mosquito data 1999-2003." Vector Borne Zoonotic Diseases 4: 360–378.
Arnett J, Ross H. 1949. "Notes on the distribution, habits, and habitats of some Panama culicines (Diptera: Culicidæ)." Journal of the New York Entomological Society 57: 233–251.
Carpenter SJ, LaCasse WJ. 1955. Mosquitoes of North America (North of Mexico), [California library reprint series edition 1974]. Berkeley and Los Angeles, CA: University of California Press.
Chamberlain RW, Sikes RK, Nelson DB. 1956. "Infection of Mansonia perturbans and Psorophora ferox mosquitoes with Venezuelan equine encephalomyelitis virus." Proceedings of the Society for Experimental Biology and Medicine 91: 215–216.
Coimbra TLM, Santos RN, Petrella S, Nagasse-Sugahara TK, Castrigano SB, Santos CLS. 2008. "Molecular characterization of two Rocio flavivirus strains isolated during the encephalitis epidemic in São Paulo state, Brazil and the development of a one-step RT-PCR assay for diagnosis." Revista do Instituto de Medicina Tropical de São Paulo 50: 89–94.
Cutwa MM, O'Meara GF. 2005. Photographic Guide to Common Mosquitoes of Florida. Gainesville: University of Florida Institute of Food and Agricultural Sciences.
Darsie RF, Ward RA. 2005. Identification and Geographical Distribution of the Mosquitos of North America, North of Mexico, [2nd ed., rev.]. Gainesville, FL: University Press of Florida.
Diaz LA, Spinsanti LI, Almiron WR, Contigiani MS. 2003. "Una virus: First report of human infection in Argentina." Revista do Instituto de Medicina Tropical de São Paulo 45: 109–110.
Gillett JD. 1972. The Mosquito. Garden City, NY: Doubleday and Company.
Grabham M. 1903. "Four new Culicidae from Jamaica, West Indies." Canadian Entomologist 38: 311–320.
Johnson BW, Cruz C, Felices V, Espinozoa WR, Mancok SR, Guevara C, Olson JG, Kochel TJ. 2007. "Ilheus virus isolate from a human, Ecuador." Emerging Infectious Disease 13: 956–958.
King WV, Bradley GH, McNeel TE. 1942. The mosquitoes of the southern states. United States Department of Agriculture Miscellaneous Publication Number 336. [Revised] 96 pp.
Kulasekera VL, Kramer L, Nasci RS, Montashari F, Cherry B, Trock SC, Glaser C, Miller JR. 2001. "West Nile virus infection in mosquitoes, birds, horses, and humans, Staten Island, New York, 2000." Emerging Infectious Disease 7: 722–725.
Linley JR, Chadee DD. 1990. "Fine-structure of the eggs of Psorophora columbiae, Psorophora cingulata and Psorophora ferox (Diptera: Culicidae)." Proceedings of the Entomological Society of Washington 92: 497–511.
Lopes ODS, Sacchetta LDA, Francy DB, Jakob WL, Calisher CH. 1981. "Emergence of a new arbovirus disease in Brazil." American Journal of Epidemiology 113: 122–125.
Magnarellli LA. 1980. "Bionomics of Psorophora ferox (Diptera: Culicidae): Seasonal occurrence and acquisition of sugars." Journal of Medical Entomology 17: 328–332.
Micieli M. 2003. "Life cycle and epizootiology of Amblyospora ferocis (Microspora: Amblyosporidae) in the mosquito Psorophora ferox (Diptera: Culicidae)." Folia Parasitologica 50: 171–175.
Mitchell CJ, Monath TP, Cropp CB. 1981. "Experimental transmission of Rocio virus by mosquitoes." American Journal of Tropical Medicine and Hygiene 30: 465-472.
Molaei G, Andreadis TG, Armstrong PM, Diuk-Wasser M. 2008. "Host-feeding patterns of potential mosquito vectors in Connecticut, USA: Molecular analysis of bloodmeals from 23 species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia." Journal of Medical Entomology 45: 1143–1151.
Nielsen HT. 1964. "Swarming and some other habits of Mansonia perturbans and Psorophora ferox (Diptera: Culicidae)." Behaviour 24: 67–89.
Turell MJ, O'Guinn ML, Jones JW, Sardelis MR, Dohm DJ, Watts DM, Fernandez R, Da Rosa AT, Guzman H, Tesh R, Rossi CA, Ludwig GV, Mangiafico JA, Kondig J, Wasieloski Jr LP, Pecor J, Zyzak M, Schoeler G, Mores CN, Calampa C, Lee JS, Klein TA. 2005. "Isolation of viruses from mosquitoes (Diptera: Culicidae) collected in the Amazon Basin region of Peru." Journal of Medical Entomology 42: 891–989.
Weaver SC, Ferro C, Barrera R, Boshell J, Navarro JC. 2004. Venezuelan equine encephalitis. Annual Review of Entomology 49: 141–174.
WRBU (Walter Reed Biosystematics Unit). (2013a). Psorophora. http://www.wrbu.org/generapages/psorophora.htm (no longer available online).
WRBU (Walter Reed Biosystematics Unit). (2013b). Psorophora ferox. (12 August 2015).