The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The Zygaenidae (burnets and smoky moths) are small, mostly day-flying moths, although Neoprocris floridana has also been collected at night at black lights by John Heppner (Tarmann 1984a, p. 77). At least three zygaenid subfamilies (Zygaeninae, Procridinae, and Chalcosiinae) appear to be monophyletic (Epstein et al. 1999). General reviews of the characteristics and biology of the Zygaenidae and subfamilies are provided by Epstein and Adams (2009), Ebert et al. (1994), Epstein et al. (1999) and Tarmann (2004). All native New World zygaenids belong to the subfamily Procridinae.
The typical resting position of zygaenids is with the wings folded back and roof-like over the abdomen (Figure 1).

Credit: Donald W. Hall, University of Florida
The family Zygaenidae is best known for the spectacular colors of some of the Palearctic species, particularly those belonging to the genus Zygaena. Common name terminology for zygaenids is not uniform. However, brightly-colored species of the genus Zygaena are usually known as burnets while the less colorful species of Zygaena and the Old World Procridinae are often called foresters. Our eastern U.S. species are known as either smoky moths or skeletonizers (after the feeding habits of early instars).
The following five species of zygaenids besides Neoprocris floridana occur in the eastern US:
Harrisina americana (Guérin 1829)—grapeleaf skeletonizer moth
Acoloithus falsarius Clemens 1860—Clemens' false skeletonizer
Acoloithus novaricus Barnes & McDunnough, 1913—no common name
Pyromorpha dimidiata (Herrich-Schäffer, 1854)—orange-patched smoky moth
Pryeria sinica Moore, 1877—Euonymous defoliator moth (Introduced from Japan and now established in Virginia and possibly also Maryland).
Adults of the first four species can be differentiated from Neoprocris floridana by the presence of some orange color on the prothorax (Harrisina americana, Acoloithus falsarius, and Acoloithus novaricus) or wings (Pyromorpha dimidiata). Neoprocris floridana contains no orange (or yellow). The introduced species, Pryeria sinica, has clear wings. For color photographs, distribution maps, and seasonal data for these species, see their respective species pages at the North American Moth Photographers Group website (http://mothphotographersgroup.msstate.edu/).
Some tiger moths, the yellow-collared scape moth (Cisseps fulvicollis [Hübner]), and the black-winged Dahana moth (Dahana atripennis Grote) (Erebidae: Arctiinae: Arctiini: Ctenuchina), resemble zygaenids. However, both moths have some orange or yellow coloration which distinguishes them from Neoprocris floridana. Also, tiger moths have only a single complete front wing anal vein while zygaenids have two (Triplehorn and Johnson 2005) (Figure 2).

Credit: Donald W. Hall, University of Florida
Distribution
Neoprocris floridana occurs throughout Florida except for the Keys (North American Moth Photographers Group) and is also reported from Lee County, Alabama (Hyche 1998). Its host plant, Carolina laurelcherry (Prunus caroliniana [Mill.] Aiton) (Rosaceae), is native to the coastal states from North Carolina to Texas and also Arkansas (Plants Database 2014). It is likely that Neoprocris floridana is present in other southeastern coastal states where its host plant is found.
Description
Eggs
The light yellow eggs are flattened and slightly rectangular (approximately 0.6 × 0.45mm) (Figure 3). The upper surface is irregular, but many appear to have a central crater bounded by a ridge on each side. The chorion is microscopically reticulated (Figure 3 [inset a]).

Credit: Donald W. Hall, University of Florida
Larvae
First and second instars are yellow (Figure 4). Later instars have a pattern of dark lines.

Credit: Donald W. Hall, University of Florida
Full-grown larvae are approximately 1.3 cm in length (approximately ½ inch). Dorsal areas are white with black mid-dorsal and dorso-lateral chain-like patterns extending from the mesothorax down the length of the body. Lateral areas of the body are yellow. Dorsal, subdorsal, lateral, and ventral verrucae bear venomous setae (Figure 5 [inset a]). The head is retractile and covered by the hood-like prothorax (Figure 5 [inset b]). There are paired, sclerotized areas of unknown function (possibly exocrine) directly behind the dorsal mesothoracic verrucae (Figure 5 [inset c]). These structures have not been reported from other zygaenid larvae.

Credit: Donald W. Hall, University of Florida
All procridine larvae are reported to have elliptical, semi-eversible, gland-like structures of unknown function just beneath the spiracles of abdominal segments two and seven (Efetov and Tarmann 2004, Stehr 1987, Veglialante and Hasenfuss 2012 [Supplemental Figure 4d]). In Neoprocris floridana, the cuticle of these glands is not well-differentiated from the surrounding cuticle. I have been able to observe them in full-grown larvae only when squeezing the larvae with forceps to cause the glands to evert.
Cocoons and Pupae
Cocoons are flattened and approximately 1.0 × 0.5 cm (approximately 0.4 × 0.2 in). The bottom of the cocoons (the surface in contact with the substrate) is thin and papery. The upper surface is tough. Calcium oxalate monohydrate raphides (needles) produced in the Malpighian tubules are excreted through the anus and incorporated as small clumps into the outer web-like layer of silk (Naumann 1977, Ohnishi et al. 1968) (Figure 6).
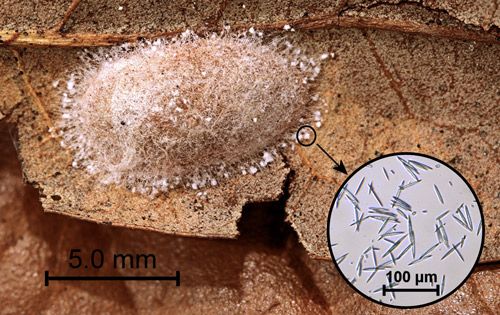
Credit: Donald W. Hall, University of Florida
The quantity of calcium oxalate raphides varies considerably. The entire surface of some cocoons is nearly covered giving them a white appearance (Figure 7).

Credit: Donald W. Hall, University of Florida
Pupae are moderately sclerotized (Figure 8) and dorso-ventrally flattened. Appendages are weakly attached to the body (Epstein et al. 1999), and abdominal tergites 3–7 (females) and 3–8 (males) bear transverse rows of spines anteriorly (Tarmann 2004) (Figure 8 [inset a]). Abdominal segments 3–7 in the male and 3–6 in the female are movable (Tarmann 2004), which facilitates protrusion of the pupa from the cocoon prior to adult eclosion. The cremaster is composed of six hooked setae (less commonly five). The setae are oriented with the hook point upward (Figure 8 [inset b]).

Credit: Donald W. Hall, University of Florida
Adults
Adults are black with scales on the body and antennae that iridesce bright blue (Figure 9). Females are typically slightly larger than males. Tarmann (1984a, p. 78) reported wingspreads for females of 16–21 mm (~2/3–7/8 in) and for males of 15–18 mm (~5/8–3/4 in). Also, the abdomens of females are larger.

Credit: Donald W. Hall, University of Florida
Antennae are bipectinate and taper to points. Pectinations (pectin teeth) of male antennae are approximately twice the length of those of females (Figure 10). Male pectinations have numerous sensory hairs which likely function in detection of sex pheromone.

Credit: Donald W. Hall, University of Florida
The frenulum of male Procridinae consists of a single bristle (Tarmann 2004). Tarmann (1984a [p.16] and 1984b [p. 67, Figure 226]) reported that the frenulum of female Neoprocris consists of three bristles.
However, of 25 female Neoprocris floridana of the overwintering generation from Gainesville, Florida, that I examined, only two had frenula with three bristles. All others had only two bristles (Figure 11). It is possible that females from different populations or different generations of the same population vary with respect to the number of bristles in the frenulum.
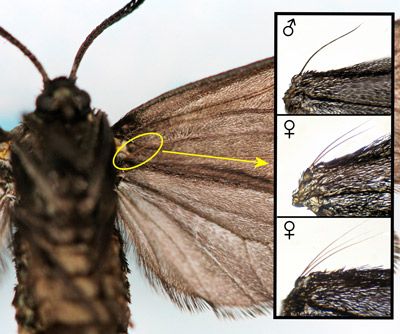
Credit: Donald W. Hall, University of Florida
Chaetosemata of Procridinae are separated (divided) in contrast to those of the other subfamilies which are fused mediodorsally (Tarmann 2004) (Figure 12).

Credit: Donald W. Hall, University of Florida
Host Plant
The only known larval host plant for Neoprocris floridana is Carolina laurelcherry, Prunus caroliniana Aiton (Rosaceae) (Tarmann 1992), and there are no other zygaenids known to feed on this species. Carolina laurelcherry is a small evergreen tree with shiny, dark green, leathery leaves (Figure 13).
The margins of leaves of older trees are usually entire (smooth), but the leaf margins of younger plants are often serrated (Figure 13 [inset]). The small white flowers are borne in the leaf axils on 2–3 inch racemes in February and March and the resulting shiny black cherries (Figure 13) are eaten by birds which then spread the seeds causing the plant to be somewhat weedy. For general information on Carolina laurelcherry, see Godfrey (1988), Haehle and Brookwell (1999), and Nelson (2003).

Credit: Donald W. Hall, University of Florida
Because of its attractive foliage, Carolina laurelcherry has been used as an ornamental or for hedges and screens in home landscapes (Figure 14).

Credit: Donald W. Hall, University of Florida
Life Cycle and Biology
There are three generations per year with adults reported from February–April, July, and September–October (Heppner 2003). Winter diapause is spent in the pupal stage (personal observation).
Prior to adult eclosion, the pupa (pharate adult) forces its way out of the cocoon (dorsal side up) by repeated, sequential lengthening and shortening of the abdomen while hooking the dorsal bands of spines of the abdominal segments into the cocoon to move itself forward. When all but the terminal segments of the abdomen are out of the cocoon, the adult emerges. The pupal exuviae is left protruding from the cocoon (Figure 15) anchored by the cremaster.

Credit: Donald W. Hall, University of Florida
Adult zygaenids have a well-developed proboscis and visit flowers. I have been unable to observe Neoprocris floridana feeding on native flower species, but Joe Cicero (personal communication) observed one feeding on a flower of exotic Indian mustard, Brassica juncea (L.) Czern. (Brassicaceae).
Pheromones and Mating
The position of the pheromone glands in Procridinae is unusual. While sex pheromone glands of most Lepidoptera are in the terminal segments of the abdomen, those of procridines are under the anterior edges of the 3rd–5th tergites (Hallberg and Subchev 1997, Subchev 2014) and are connected to the outside by ducts to setae. The pheromone is believed to be released through pores in the setae (Hallberg and Subchev 1997). To release the pheromone, procridines have a unique calling posture with the wings spread downward and the abdomen bowed to expose the anterior edges of tergites 3–5 (Hallberg and Subchev 1997) (Figure 16) which would normally be covered by the posterior edges of the preceding tergites.

Credit: Donald W. Hall, University of Florida
Some Procridinae use enantiomers (stereoisomers that are mirror images of each other and non-superimposable on one another) of 2-butyl-(Z)-7-tetradecenoate as sex pheromones (Curtis et al. 1989, Landolt and Heath 1987, Landolt and Heath 1991, Landolt et al. 1986, Subchev 2014, Subchev et al. 2010). Neoprocris aversa (Edwards), a southwestern US species has been shown to use the (R) enantiomer of 2-butyl-(Z)-9-tetradecenoate (Subchev 2014). The pheromone of Neoprocris floridanahas not been identified.
Males attracted to caged females flutter around with their claspers spread (Figure 17). Caged virgin females are attractive to males throughout most of the photophase except in February during time periods when temperatures are too low for activity. Calling throughout the entire photophase has been previously reported for at least two Palearctic procridines, Theresimima ampellophaga (Bayle-Barelle) and Rhagades pruni Denis & Schiffenmuller (Toshova and Subchev 2005).

Credit: Donald W. Hall, University of Florida
Mating is end-to-end with the male's wings on top (Figure 18).

Credit: Donald W. Hall, University of Florida
Eggs are usually laid in large clusters on the undersides of young leaves near the leaf margins (Figure 19).

Credit: Donald W. Hall, University of Florida
Eggs hatch in approximately 10 days. Larvae eclose by chewing through the chorion at the end of eggs (Figure 2, [inset b]). Sometimes (but not always), they eat the chorions of the eggs. Early instars are leaf skeletonizers (Figure 20, left). Later instars feed on leaf edges (Figure 20, right) and sometimes completely defoliate host plants (Figure 21) including mature trees. Newly-molted larvae of all instars conserve nutrients by eating their exuviae (Figure 4).

Credit: Donald W. Hall, University of Florida

Credit: Donald W. Hall, University of Florida
Full-grown larvae spin cocoons on dead leaves in the litter beneath the host plants.
Medical Importance
Diaz (2005) listed Zygaenidae (including Neoprocris spp.) among the families of Lepidoptera with envenoming caterpillars. Zygaenid caterpillars, including those of Neoprocris floridana have mildly venomous setae. The setae are borne on prominent sub-dorsal, lateral, sub-spiracular and ventral verrucae on the meso- and meta-thorax and abdominal segments one through eight (Figure 5). Each venomous seta has a basal bulb and is annulated throughout its length (Figure 5 [inset a]). The setae appear identical to those of the Oriental procridine Artona funeralis Butler described by Tsutsumi (1959) who found that the setae were hollow and filled with a yellow urticating fluid. In contrast to stings by caterpillars of most other lepidopteran families, the tips of the venomous setae of Artona were not broken off and left embedded in the skin.
I pressed a full-grown caterpillar against my inner wrist and within a minute I experienced a mild, burning itch followed by erythema (reddening) in the area contacted by the caterpillar. Within five minutes a raised wheal (blister) appeared covering the area contacted by the caterpillar (Figure 22). The burning itch was completely gone within an hour. By two hours, the wheal had disappeared, but the area was still erythematous.
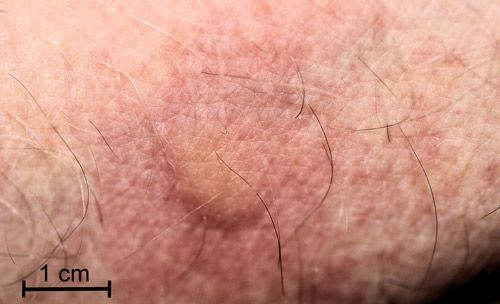
Credit: Donald W. Hall, University of Florida
Natural Enemies
Parasitoids
The only parasitoids known from Neoprocris floridana are tachinid flies. Patton (1958, p. 80) reported Phorocera subnitens Aldrich & Webber (Tachinidae) from a moth larva identified as "near Triprocris" feeding on laurelcherry. The moth larva was certainly Neoprocris floridana. O'Hara (2013) listed the tachinid under its current name, Chetogena subnitens (Aldrich & Webber 1924).
Of overwintering Neoprocris floridana cocoons which yielded live moths or flies (N=204), 23 (11.3%) were parasitized by tachinids. Because of their small size, each Neoprocris floridana will support development of a single tachinid. The tachinid life cycle is shown in Figure 23. The tachinid egg is laid near the front of the host larva's body where the larva cannot reach it to remove it. After hatching, the tachinid larva penetrates the integument of the Neoprocris floridana larva but maintains a respiratory funnel to the outside which it also maintains in the Neoprocris floridana pupa. The tachinid larva completes its development within the Neoprocris floridana pupa and usually pupates within the host pupal exoskeleton inside the host cocoon. Subsequently, the adult fly breaks out of its puparium, through the host pupal exoskeleton, and forces its way out of the host's cocoon (probably by using its ptilinum).
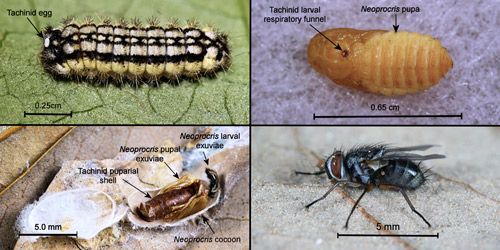
Credit: Donald W. Hall, University of Florida
Predators
Laurelcherry smoky moths are likely chemically-defended from most vertebrate predators. Epstein and Adams (2009) stated that zygaenid moths are avoided by birds and other predators. However, I have fed them to exotic Cuban brown anoles, Anolis sagrei Dumeril and Bibron (Reptilia: Polychrotidae), and the anoles did not exhibit any aversion or short term signs of poisoning.
I have fed full-grown larvae to a wheel bug, Arilus cristatus (Linnaeus) (Reduviidae) which ate them without hesitation and showed no signs of poisoning. Insects are probably the major predators of zygaenids.
Defenses
Avoidance
Neoprocris floridana larvae and adults may avoid natural enemies by aposematic (warning) coloration. The yellow and white coloration of larvae with contrasting black lines (Figure 5) makes them readily recognizable to predators that may have previously had a toxic reaction to one of them.
The bright iridescence of the abdomens of adults (Figure 9), visible while the moths are in flight, may serve as a warning to potential predators. Warning coloration is proposed to be one of the functions of iridescence in animals (Doucet and Meadows 2009).
Many zygaenids are members of mimicry rings (Arillo et al. 2005). A mimicry ring is composed of a group of species that display a common warning coloration pattern and some or all of the species are unpalatable (Mallet and Gilbert 1995). Smoky moths may be members of Müllerian mimicry rings (all species in group are unpalatable) involving wasps or other chemically defended insects. In flight, smoky moths resemble dark-colored wasps. The shiny iridescence of Neoprocris floridana may enhance this resemblance.
Zygaenidae is one of at least six families of Lepidoptera in which larvae, when disturbed, drop toward the ground on "lifelines" of silk thread (Sugiura and Yamazaki 2006) presumably to escape predators. Neoprocris floridana commonly displays this behavior (Figure 24), and as a result, I commonly find them on my clothing after collecting from their host plants. Also, this behavior is common when a branch of the host plant or the entire plant has been defoliated and larvae have no food (personal observation). The wind may blow the silk lifelines or the larvae against lower branches or adjacent plants with leaves. Dozens of suspended larvae may sometimes be observed hanging from a single defoliated host tree.

Credit: Donald W. Hall, University of Florida
Host feces may serve as an arrestant or attractant for parasitoids (Afsheen et al. 2008, Godfray 1994, Stireman et al. 2002, Tanaka et al. 2001). Larvae from at least 17 families of Lepidoptera forcibly eject their fecula (fecal pellets) (Weiss 2003). Over one third of the families with species that eject their feces (including the Zygaenidae) belong to the superfamily Zygaenoidea (Epstein 1996). Larvae that forcibly eject their fecula have anal combs or forks that serve as latches in a pressure driven system to achieve the ejection (Caveny et al. 1998). These combs or forks are usually located above the anus on the underside of the anal plate and vary in morphology between species of zygaenids (Efetov 2004).
The anal comb of Neoprocris floridana consists of a row of stout spines (Figure 25). However, because the larvae spend most of their time on the edges or undersides of leaves and only eject their feces several centimeters (D.W. Hall personal observation), fecal ejection may not serve an adaptive function in this species.

Credit: Donald W. Hall, University of Florida
Chemical Defenses
All stages of Zygaenidae contain the cyanogenic ß–glucosides linamarin and lotaustralin (Zagrobelny et al. 2007, 2008), and many species are extremely resistant to poisoning by cyanide. For chemical information on these compounds see: http://pubchem.ncbi.nlm.nih.gov/compound/11128 (linamarin) and http://pubchem.ncbi.nlm.nih.gov/compound/441467 (lotaustralin)
Linamarin and lotaustralin undergo enzymatic breakdown to release toxic, free hydrogen cyanide (HCN) as a defense when zygaenids are attacked. Cyanogenic glucosides may be sequestered from host plants and/or synthesized de novo (Fürstenberg-Hägg et al. 2014).
It has been shown in Zygaena filipendulae (Linnaeus) (Zygaenidae: Zygaeninae) that males transfer these compounds to females as nuptial gifts, that females mate preferentially with males that have a higher content of cyanogenic glucosides, and that males may be attracted to females that emit high concentrations of HCN (Zagrobelny et al. 2007, 2008, 2014). It is unknown whether or not this is true for Neoprocris species.
The foliage of laurelcherry has high concentrations of cyanogenic glucosides (Hall et al. 1969, Nelson 2003) which are likely sequestered by Neoprocris floridana larvae. The concentration of cyanide in foliage is sufficiently high that Hall et al. (1969) suggested using crushed laurelcherry leaves as the toxicant for insect killing jars. They charged four ounce jars with five mg. of crushed leaves each and recorded knockdown times for 11 species of insects. Examples of times required for 100% knockdown were nine seconds for the mosquito Anopheles quadrimaculatus Say (Diptera: Culicidae), 12 seconds for the honey bee, Apis mellifera Linnaeus (Hymenoptera: Apidae), and 135 seconds for the squash bug Anasa tristis (De Geer) (Hemiptera: Coreidae).
Female Zygaeninae cover their eggs with a thin layer of proteinaceous material of glandular origin (Petersen's glands) that is believed to be poisonous and protect the eggs from parasitoids (Tarmann 2004). Similar pairs of glands, believed to be homologous to Petersen's glands, occur in the Procridinae (Tarmann 2004).
The stinging setae of older larvae (Figure 5 [inset a]) may serve to protect them from vertebrate predators, but are probably ineffective against most insect predators.
The calcium oxalate monohydrate raphides in the outer webbing layer of Neoprocris floridana cocoons (Figures 6 & 7) almost certainly serve a defensive function against potential predators attempting to chew into the cocoon. Raphides are common in plants and are increasing believed to have a defensive function against herbivores (Finley 1999, Hanley et al. 2007, Konno et al. 2014, Nakata 2003). Neoprocris floridana larvae spend considerable time and effort distributing the raphides. First they excrete the oxalate at one end of the early cocoon and turn around and appear to add material to it with the mouthparts. Next they work it into the silk webbing—first on one side of the cocoon and then the other with their mouthparts (Figure 26). The behavior is then repeated at the other end of the cocoon.
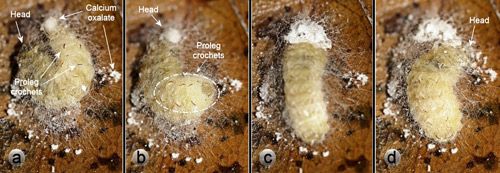
Credit: Donald W. Hall, University of Florida
When disturbed, adults release a droplet of fluid from a pore between the inner margin of the compound eye and the base of the proboscis (Figure 27) (Epstein et al. 1999). Even grasping a wing with forceps will elicit release of the droplet by adults of Neoprocris floridana. The function of the droplet is unknown but may be defensive.
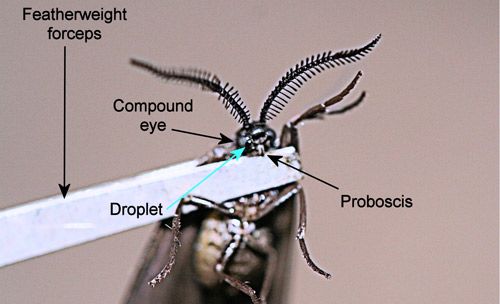
Credit: Donald W. Hall, University of Florida
Management
The laurelcherry smoky moth is not economically important. Although it is common on individual plants, I have not seen damage to landscape hedges. Regular pruning of the hedges may interrupt its life cycle. If control measures are required, Bacillus thuringiensis applications or chemical insecticides recommended for control of other caterpillars should be effective. For current control recommendations, contact your County Extension Office.
Acknowledgement
The author would like to acknowledge Andrei Sourakov for reviewing this article and offering helpful suggestions.
Selected References
Afsheen S, Wang X, Li R, Zhu C-S, LouY-G. 2008. Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect Science 15: 381-397. https://doi.org/10.1111/j.1744-7917.2008.00225.x
Aldrich JM, Webber RT. 1924. The North American species of parasitic two-winged flies belonging to the genus Phorocera and related genera. Proceedings of the United States National Museum 63 (Art. 17) [= No. 2486]: 1-90. https://doi.org/10.5479/si.00963801.63-2486.1
Arillo A, Balletto E, Cassulo L. 1992. Melanism, mimicry and chemical deterrence in zygaenid moths (Lepidoptera: Zygaenidae). Theses Zoologicae 19: 9-20.
Caveny S, McLean H, Surry D. 1998. Faecal firing in a skipper butterfly is pressure-driven. Journal of Experimental Biology 201: 121-133. https://doi.org/10.1242/jeb.201.1.121
Curtis CE, Landolt PJ, Heath RR. 1989. Attraction of western grapeleaf skeletonizer males (Lepidoptera: Zygaenidae) to S-(+)-2-butyl-(Z)-7-tetradecenoate. Journal of Economic Entomology 82: 454-457. https://doi.org/10.1093/jee/82.2.454
Diaz JH. 2005. The evolving global epidemiology, syndromic classification, management, and prevention of caterpillar envenoming. American Journal of Tropical Medicine and Hygiene 72: 347-357. https://doi.org/10.4269/ajtmh.2005.72.347
Doucet SM, Meadows MG. 2009. Iridescence: A functional perspective. Journal of the Royal Society Interface (Interface Focus Supplement) 6: S115-S132. https://doi.org/10.1098/rsif.2008.0395.focus
Ebert G, Hofmann A, Lussi HG. 1994. Zygaenidae. In Ebert G, Rennwald E, eds. Die Schmetterlinge Baden-Württembergs 3: 153-335. E. Ulmer. Stuttgart, Germany.
Efetov KA. 2004. Anal combs. pp. 183-189. In Efetov KA. Forester and Burnet Moths (Lepidoptera: Zygaenidae). Crimean State Medical University Press. Simferopol, Crimea, Ukraine. 272 pp.
Efetov KA, Tarmann GM. 2004.The presence of lateral abdominal 'glands' in some species of Zygaenidae (Insecta, Lepidoptera). Denisia 13: 301-303.
Epstein ME. 1996. Revision and phylogeny of the limacodid-group families, with evolutionary studies on slug caterpillars (Lepidoptera: Zygaenoidea). Smithsonian Contributions to Zoology. Number 582. 102 pp. https://doi.org/10.5479/si.00810282.582
Epstein ME, Adams JK. 2009. Superfamily Zygaenoidea. pp. 160-165 In Powell JA, Opler PA. Moths of Western North America. University of California Press. Berkeley, California. 383 pp.
Epstein ME, Geertsema H, Naumann CM, Tarmann GM. 1999. Chap. 10. The Zygaenoidea. Handbook of Zoology (Berlin) 4: 159-180. https://doi.org/10.1515/9783110804744.159
Finley DS. 1999. Patterns of calcium oxalate crystals in young tropical leaves: a possible role as an anti-herbivory defense. Revista de Biología Tropical 47: 27-31. https://doi.org/10.15517/rbt.v47i1-2.18998
Fürstenberg-Hägg J, Zagrobelny M, Olsen CE, Jørgensen K, Møller BL, Bak S. 2014. Transcriptional regulation of de novo biosynthesis of cyanogenic glucosides throughout the life-cycle of the burnet moth Zygaena filipendulae (Lepidoptera). Insect Biochemistry and Molecular Biology 49: 80-89. https://doi.org/10.1016/j.ibmb.2014.04.001
Godfray HCJ. 1994. Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press. Princeton, New Jersey. 473 pp. https://doi.org/10.1515/9780691207025
Godfrey RK. 1988. Trees, Shrubs, and Woody Vines of Northern Florida and Adjacent Georgia and Alabama. The University of Georgia Press. Athens, Georgia. 734 pp.
Haehle RG, Brookwell J. 1999. Native Florida Plants: Low-maintenance Landscaping and Gardening. Gulf Publishing Company. Houston, Texas. 360 pp.
Hall TF, Breeland SG, Anderson PK. 1969. Use of cherry-laurel foliage for preparation of an effective insect-killing jar. Annals of the Entomological Society of America. 62: 242-244. https://doi.org/10.1093/aesa/62.1.242
Hallberg E, Subchev M. 1997. Unusual location and structure of female pheromone glands in Theresimima(=Ino) ampelophaga Bayle-Berelle (Lepidoptera: Zygaenidae). International Journal of Insect Morphology and Embryology 25: 381-389. https://doi.org/10.1016/S0020-7322(96)00017-7
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. 2007. Plant structural traits and their role in anti-herbivory defense. Perspectives in Plant Ecology, Evolution and Systematics 8: 157-178. https://doi.org/10.1016/j.ppees.2007.01.001
Heppner JB. 2003. Lepidoptera of Florida. Part 1. Introduction and Catalog. Volume 17 of Arthropods of Florida and Neighboring Land Areas. Division of Plant Industry. Florida Department of Agriculture and Consumer Services. Gainesville, Florida. 670 pp. https://doi.org/10.64338/fsca.af.17.q8t22
Hyche LL. 1998. Stinging caterpillars: A guide to recognition of species found on Alabama trees.
Konno K, Inoue TA, Nakamura M. 2014. Synergistic defensive function of raphides and protease through the needle effect. PLoS ONE 9: e91341. https://doi.org/10.1371/journal.pone.0091341
Landolt PJ, Heath RR. 1987. Seasonal and diel patterns of sex attraction of male Harrisina americana andAcoloithus falsarius (Lepidoptera: Zygaenidae). Florida Entomologist 70: 392-396.
Landolt PJ, Heath RR. 1991. Zygaenidae trapped with enantiomers of 2-butyl(Z)-7-tetradecenoate. Journal of the Lepidopterists' Society 45: 63-65.
Landolt PJ, Heath RR, Sonnet PE, Matsumoto K. 1986. Attraction of Harrisina americana and Acoloithus falsarius males (Lepidoptera, Zygaenidae) to (R)-(-)-2-Butyl-(Z)-7-tetradecenoate. Environmental Entomology 15: 959-962. https://doi.org/10.1093/ee/15.4.959
Mallet J, Gilbert LE Jr. 1995. Why are there so many mimicry rings? Correlations between habitat, behavior and mimicry in Heliconius butterflies. Biological Journal of the Linnean Society 55: 159-180. https://doi.org/10.1111/j.1095-8312.1995.tb01057.x
Nakata PA. 2003. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Science 164: 901-909. https://doi.org/10.1016/S0168-9452(03)00120-1
Naumann CM. 1977. Rasterelektronenoptische Untersuchungen zur Feinstruktur von Lepidopteren-Gespinsten. Mitteilungen der Münchner Entomologischen Gesellschaft 67: 27-37.
Nelson G. 2003. Florida's Best Native Landscape Plants: 200 Readily Available Species for Homeowners and Professionals. University Press of Florida. Gainesville, FL. 411 pp.
North American Moth Photographers Group (Neoprocris floridana page).
O'Hara JE. 2013. Genus Chetogena Rondani, 1856. Taxonomic and Host Catalogue of the Tachinidae of America North of Mexico.
Ohnishi E, Takahashi SY, Sonobe H, Hayashi T. 1968. Crystals from cocoons of Malacosoma Neustria testacea. Science 160:783-784. https://doi.org/10.1126/science.160.3829.783
Patton, C. N. 1958. A catalog of the Larvaevoridae of Florida. Florida Entomologist 41: 29-39 & 41: 77-89. https://doi.org/10.2307/3492364
Plants Database. 2014. USDA Natural Resources Conservation Service. http://plants.usda.gov
Stehr FW. 1987. Zygaenidae (Zygaenoidea). pp. 453-454. In Stehr FW. (ed.). Immature Insects. Kendall/Hunt. Dubuque, Iowa. 768 pp.
Stireman, JO. 2002. Host location and selection cues in a generalist tachinid parasitoid. Entomologia Experimentalis et Applicata 103: 23-34. https://doi.org/10.1046/j.1570-7458.2002.00958.x
Subchev M. 2014. Sex pheromone communication in the family Zygaenidae (Insecta: Lepidoptera): A review. Acta Zoologica Bulgarica 66: 147-157.
Subchev M, Efetov KA, Toshova T, Parshkova EV, Toth M, Francke W. 2010. New sex attractants for species of the zygaenid subfamily Procridinae (Lepidoptera: Zygaenidae). Entomologia Generalis 32: 243-250. https://doi.org/10.1127/entom.gen/32/2010/243
Sugiura S, Yamazaki K. 2006. The role of silk threads as lifelines for caterpillars: pattern and significance of lifeline-climbing behavior. Ecological Entomology 31: 52-57. https://doi.org/10.1111/j.0307-6946.2006.00755.x
Tanaka C, Kainoh Y, Honda H. 2001. Host frass as arrestant chemicals in locating host Mythimna separata by the tachinid fly Exorista japonica. Entomologia Experimentalis et Applicata 100: 173-178. https://doi.org/10.1046/j.1570-7458.2001.00861.x
Tarmann G. 1984a. Generische Revision der amerikanischen Zygaenidae mit Beschreibung neuer Gattungen und Arten (Insecta: Lepidoptera). Teil 1: Text. Entomofauna Supplement 2: 1-176.
Tarmann G. 1984b. Generische Revision der amerikanischen Zygaenidae mit Beschreibung neuer Gattungen und Arten (Insecta: Lepidoptera). Teil 2: Figures. Entomofauna Supplement 2: 1-153.
Tarmann G. 1992. Foodplants of the Zygaenidae subfamilies Procridinae and Chalcosiinae, with notes on the biology and ecology of these two groups. Theses Zoologicae 19: 144-161.
Tarmann G. 2004. Zygaenid Moths of Australia: A Revision of the Australian Zygaenidae (Procridinae: Artonini). CSIRO Publishing. Collingwood, Australia. 248 pp. https://doi.org/10.1071/9780643092198
Toshova T, Subchev M. 2005. Circadian rhythm of female calling and mating in Procridinae (Lepidoptera: Zygaenidae). Acta Entomologica Bulgarica 11: 57-64.
Triplehorn CA, Johnson NF. 2005. Borror and Delong's Introduction to the Study of Insects. Thomson – Brooks/Cole. Belmont, California. 7th edition. 864 pp.
Tsutsumi C. 1959. Structure, development and sting mechanism of the larval poison hair of Artona funeralisButler (Lepidoptera: Zygaenidae). Japanese Journal of Medical Science and Biology 12: 421-428. https://doi.org/10.7883/yoken1952.12.421
Vegliante F, Hasenfuss I. 2012. Morphology and diversity of exocrine glands in lepidopteran larvae. Annual Review of Entomology 57: 187-204. Supplements: http://www.annualreviews.org/doi/suppl/10.1146/annurev-ento-120710-100646. https://doi.org/10.1146/annurev-ento-120710-100646
Weiss MR. 2003. Good housekeeping: why do shelter-dwelling caterpillars fling their frass? Ecological Letters 6: 361-370. https://doi.org/10.1046/j.1461-0248.2003.00442.x
Zagrobelny M, Olsen CE, Bak S, Møller BL. 2007. Intimate roles for cyanogenic glucosides in the life cycle of Zygaena filipendulae (Lepidoptera, Zygaenidae). Insect Biochemistry and Molecular Biology 37: 1189-1197. https://doi.org/10.1016/j.ibmb.2007.07.008
Zagrobelny M, Bak S, Møller BL. 2008. Cyanogenesis in plants and animals. Phytochemistry 69: 1457-1468. https://doi.org/10.1016/j.phytochem.2008.02.019
Zagrobelny M, Olsen CE, Pentold S, Fürstenberg-Hagg J, Jørgensen K, Bak S, Møller BL, Motawia, MS. 2014. Sequestration, tissue distribution and developmental transmission of cyanogenic glucosides in a specialist insect herbivore. Insect Biochemistry and Molecular Biology 44: 44-53. https://doi.org/10.1016/j.ibmb.2013.11.003