The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The spicebush swallowtail butterfly, Papilio troilus Linnaeus, is one of our most beautiful and interesting swallowtails. It is relatively common in natural areas and flower gardens throughout the eastern and parts of the midwestern United States. Larvae, pupae and adults are great examples of adaptive coloration. A male spicebush swallowtail was featured on the third butterfly stamp (issued January, 2013) in the U.S. Postal Service's series of stamps for large greeting cards that require additional postage (Figure 1).

Credit: http://about.usps.com/news/national-releases/2013/pr13_008.htm (No longer online.)
Nomenclature
Linnaeus grouped some swallowtails and other butterflies under the genus name Papilio and used the names of heroes from the Trojan War as specific epithets (Tyler 1975). Papilio is the Latin word for butterfly. The subgenus name Pterourus is from the Greek roots "ptero" for wing and "ura" for tail (Borror 1960). Troilus was the son of Priam, king of Troy, in Homer's Iliad.
There is disagreement on the generic classification of the swallowtails (Hancock 1983, Miller 1987). Some authors (e.g., Tyler et al. 1994, Minno et al. 2005, Gatrelle 2000, Warren et al. undated) follow the system that elevates the subgenus Pterourus to generic status as proposed by Hancock (1983). Because the name Papilio is still so widely used in sources available to the public, it will be used here instead of Pterourus for practical reasons.
Historically, two subspecies have been recognized: Papilio troilus troilus, which was considered to be distributed throughout the range, and Papilio troilus ilioneus, which was considered to be restricted to the southern coastal plain including the Florida peninsula. However, Gatrelle (2000) maintained that only subspecies troilus occurs south through northern Florida and that ilioneus is not a valid subspecies. He described a new subspecies fakahatcheensis from southwestern Florida, and stated that specimens from the area of middle and upper-southern Florida are intermediates of Papilio troilus troilus and Papilio troilus fakahatcheensis. Papilio troilus fakahatcheensis is now very rare and probably imperiled (Marc Minno personal communication).
Distribution
The spicebush swallowtail is found throughout the eastern half of the United States from southern Canada south to southern Florida (except the Miami area and Keys) and west to Texas. It is less common farther west from the Mississippi River. Occasional stray insects are observed outside the normal range.
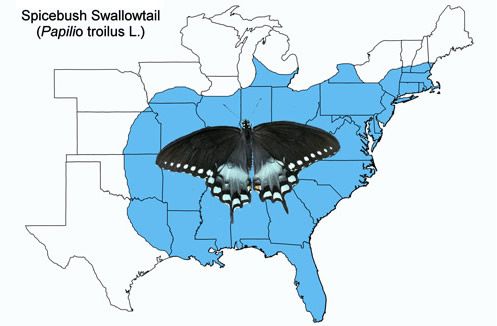
Credit: Map prepared by Donald W. Hall, UF/IFAS Department of Entomology and Nematology. (Compiled from a variety of sources.)
Description
Adults
The wingspread range is 92–124 mm (3.83–4.78 in) (Opler and Malikul 1998). The upper surface of the forewings is black with a narrow marginal row and a broader sub-marginal row of light yellowish spots. The upper surfaces of the hind wings also have the rows of spots, but they are light green in color. The median areas of the hind wings are dusted with blue in females (Figure 3) and blue-green to green in males (Figure 4). There is considerable variation in the blue-green coloration of males (Glassberg et al. 2000). The undersides of the hind wings have marginal pale green spots and also marginal and post-median rows of bright orange spots separated by black and blue patches (Figure 5).

Credit: Jerry F. Butler, UF/IFAS Department of Entomology and Nematology

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
Eggs
Recently laid eggs are spherical and greenish-white or white in color (Figure 6, left). The chorions (egg shells) are transparent, and the larvae are visible shortly before hatching (Figure 6, right).
![Figure 6 Figure 6. Eggs of the spicebush swallowtail, Papilio troilus L., on camphortree, (Cinnamomum camphora [L.] J. Presl). Left: recently laid egg. Right: egg shortly before hatching.](/image/IN1107/D3ei335u0i/D5qleab4to/D5qleab4to-2048.webp)
Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
Larvae
Early instars are brown or black usually with a white spiracular stripe that often extends dorsally on the first and eighth abdominal segments (Wagner 2005) (Figure 7).

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
Full-grown (fifth instar) larvae are up to 5.5 cm in length (approx. 2.17 inches) (Wagner 2005). Fifth instar larvae are green with a pale yellow lateral line edged beneath with a fine black line. The underside of newly molted fifth instar larvae is pale green but later turns to burgundy or pinkish-brown (Figure 8). Abdominal segments have a transverse band of six blue dots with each dot ringed by a fine black line (much thicker than those on larvae of the Palamedes swallowtail (Papilio palamedes Drury) the most closely related (Hagen and Scriber 1991, Scriber et al. 1998, Sperling 1993) and most similar of our swallowtails to the spicebush swallowtail. One dot on each side is beneath the lateral line. There is a pair of large tan false eyespots lined with black on the metathorax. The eyespots have a large black "pupil" with a white "false reflection". Larvae also have a smaller pair of tan spots dorsally on the first abdominal segment.

Credit: Jerry F. Butler, UF/IFAS Department of Entomology and Nematology
Pupae
Pupae have two anterior horns. Pupae from larvae developing under long photoperiods may be either green (Figure 9) or brown (Figure 10). All pupae from short photoperiod larvae (diapause pupae) are brown. Within the last 24 hours prior to adult emergence, the pre-adult gradually becomes visible through the transparent pupal cuticle (Figure 11).

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
Life Cycle
There are at least three generations in the Deep South (Gulf of Mexico area and peninsular Florida) with peak numbers of adults in late spring and early fall in central Florida and two generations northward (Cech and Tudor 2005, Howe 1975).
Males are reported to drink from mud puddles (Cech and Tudor 2005, Glassberg et al. 2000). More commonly, they are extracting minerals from wet soil, not from puddles (Marc Minno personal communication). Males patrol host plants and flyways to locate females (Lederhouse 1995). Courtship flights are slow with the male hovering above the female (Cech and Tudor 2005). Courtship and mating occur in the afternoon.
Eggs are laid singly on the undersides of new leaves of the host plants (Scriber 1996). Young trees are usually selected and eggs are typically laid from two to five meters (6.6 to 16.4 ft) above the ground.
In Lepidoptera eggs, a small quantity of yolk remains trapped between two of the embryonic membranes (amniotic and serosa) that remain inside the egg shells after hatching. Soon after hatching, larvae eat the egg shells (Figure 12), and the residual yolk serves as their first meal (Imms 1957). Larvae also eat their exuviae after molting to conserve nutrients (Figure 13).

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
First instar larvae chew a slit or slits (near the tips of leaves) from the leaf edges toward the midribs and spin silk across the leaves. As the silk dries, it contracts to curl the leaf edges over to make leaf shelters (Figure 14).
![Figure 14 Figure 14. Leaf shelter made by first instar spicebush swallowtail, Papilio troilus L., larva on red bay (Persea borbonia [L.] Spreng).](/image/IN1107/D3ei335u0i/D0vns339w0/D0vns339w0-2048.webp)
Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
Older larvae also spin silk mats (Figure 15) to curl leaf edges upward and together to form a leaf shelter composed of the whole leaf (Figure 16). Larvae usually hide in the leaf shelters during the daytime and to molt where birds and other predators are unlikely to see them. They come out to feed at night.
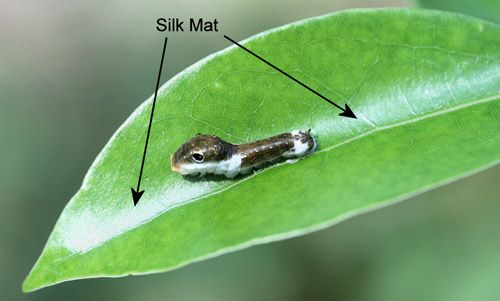
Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
Before pupation, full-grown larvae cease feeding and turn to a yellow color. These "prepupae" retain the yellow color during the pupation process (Figure 17). The prepupae wander off the host plants to pupate. Pupation is usually near the ground on slender stems among leaves (West and Hazel 1996). The color (green or brown) of non-diapausing pupae is environmentally controlled (Hazel 1995, West and Hazel 1985) by detection of the color of the pupation substrate by the stemmata (simple eyes of insect larvae) (Mellencamp et al. 2007) (Figure 13).

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
The critical photoperiod for induction of diapause in spicebush swallowtails is dependent on latitude. Valella and Scriber (2005) reported a gradient with the following extremes: 14.5-15.0 of light for southern Michigan and 12.0-12.5 for Florida populations. Pupae are the overwintering (=diapause) stage. Diapause pupae are brown.
Termination of diapause is temperature dependent regardless of photoperiod (Deering et al. 2003). Males emerge first (=protandry) (Deering et al. 2003).
Host Plants
Larval Host Plants
Spicebush swallowtail larvae are thought to feed only on plants belonging to the family Lauraceae (Minno et al. 2005, Nitao 1995, Nitao et al. 1991). Reports (e.g., Howe 1975, Robinson et al. undated, Scott 1986, Tyler 1975) of feeding on other hosts Magnolia and Liriodendron (Magnoliaceae), Prunus spp. (Rosaceae), Zanthoxylum spp. (Rutaceae), and Cercis spp. and other species of Fabaceae need further verification.
Based on laboratory feeding tests, Scriber et al. (1991) reported that neonate Papilio troilus starved to death rather than initiating feeding on non-lauraceous hosts (including sweetbay, Magnolia virginiana L.; tuliptree, Liriodendron tulipifera L.; and common pricklyash, Zanthoxylum americanum Mill.).
The following species are documented hosts:
red bay, Persea borbonia (L.) Spreng. (Figure 18)

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
swamp bay, Persea palustris (Raf.) Sarg.
sassafras, Sassafras albidum (Nutt.) Nees (Figure 19)

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
spicebush, Lindera benzoin (L.) Blume (Uncommon in Florida and occurs only in northern counties. Common further north) (Figure 20)

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
southern spicebush, Lindera melissifolia (Walter) Blume (Rare) (ref. Morris, 1989)
camphortree, Cinnamomum camphora (L.) J. Presl (Exotic—from Asia, Invasive) (Figure 21).

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
Silk bay (Persea humilis Nash) and the relatively rare pond spice (Litsea aestivalis [L.] Fernald) are also probably hosts in Florida (Minno and Minno 1999). The foliage of all of these plants is pleasingly aromatic when crushed—a characteristic that aids in differentiating them from similar plants in other families. Persea species are sometimes confused with sweetbay (Magnolia virginiana Linnaeus) but can be differentiated by the presence on sweetbay of stipular scars that completely surround the stem. Also, Persea spp. can often be identified by the characteristic leaf galls (Figure 18) caused by the red bay psyllid, Trioza magnoliae (Ashmead) (Insecta: Hemiptera: Sternorrhyncha: Psyllidae) which are almost always present.
Scriber (1996) reported that red bay is the preferred host in Florida, with spicebush and sassafras the preferred hosts in other parts of the range (Scriber 1996). However, Marc Minno (personal communication) considers camphortree and sassafras to be equally attractive. I have also found camphortree and sassafras to be highly attractive. Saplings 1–6 feet in height are most attractive to ovipositing females.
Lederhouse et al. (1992) reported that, based on three-choice laboratory tests, Papilio troilus females laid on average 11% of their eggs on Persea borbonia, 43% on Sassafras albidum, and 46% on Lindera benzoin. The percentages did not vary significantly between females from Michigan and those from Florida. Carter and Feeny (1999) and Carter et al. (1999) have identified an oviposition stimulant (3-trans-caffeoyl-muco-quinic acid) in extracts from the leaves of Sassafras albidum.
Haddad and Hicks (2000) reported that female spicebush swallowtails preferred non-pubescent to pubescent Sassafras albidum for oviposition and that the pubescent plants were suboptimal for larval development. Sassafras growing in more open habitats tends to more pubescent. Therefore, timbering operations may open the forest canopy and have negative impacts on spicebush swallowtail population numbers.
Nectar Host Plants
There are many plants that are valuable as nectar sources for butterflies. Minno and Minno (1999) have extensive lists of both native and exotic nectar plants for butterflies. When possible, native plants should be planted as nectar sources rather than exotics that have the potential to be invasive. The long proboscis of spicebush swallowtail adults allow them to feed at tubular flowers that are not accessible to many butterflies (Opler and Krizek 1984).
Most states have native plant societies that are valuable sources of information on native plants and many also hold native plant sales. For a list and contact information for native plant societies, see the American Horticultural Society website (ahsgardening.org). For Florida and the deep south, the Florida Wildflowers Growers Cooperative is an excellent source of information and also has wildflower seeds for purchase.
To maximize butterfly populations in yards, both caterpillar hosts and nectar plants for adults should be planted. Red bay, sassafras, and spicebush are recommended for spicebush swallowtail caterpillars. The choice of which one(s) to plant is dependent on locality. In areas where it will grow, red bay has the added advantage of serving as a host for caterpillars of the Palamedes swallowtail (Papilio palamedes Drury). Distribution maps for the three species can be found at the Plants National Database.
Natural Enemies
In addition to the generalist predators that prey on Lepidoptera larvae, there are at least two tachinid flies (Compsilura concinnata [Meigen] and Lespesia frenchii [Williston]) (Arnaud 1978, p. 659) and one ichneumonid wasp (Trogus pennator [Fabricius]) (Krombein et al. 1979) listed as parasitoids of spicebush swallowtails.
Defenses
The leaf shelters constructed by developing larvae likely provide some protection for all larval instars. Younger larvae (instars 1-4) are bird-dropping (or lizard-dropping) mimics (Figure 7). These instars also have false eye spots on the metathorax (third thoracic segment) (Figures 7 and 22). The white markings on the abdomens of these instars resemble the uric acid deposits in bird and lizard droppings making the resemblance even more striking.

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
The green fifth instars with their swollen thoraxes and eyespots with bulging "pupils" (Figure 8) are believed to mimic either green snakes, tree frogs (Hyla spp.) (Lederhouse 1990) (Figure 23), or lizards (Tyler et al. 1994). As Eisner et al. (2005) noted, the bulging pupils of the eyespots make the "eyes" appear to stare at you when viewed from any angle. There is a particularly striking similarity of fifth instar larvae to the squirrel tree frog, Hyla squirella Bosc. The larvae and frogs are similar in size and the brown spots on the first abdominal segment of the larvae resemble the brown tympana of the frogs.

Credit: a) Donald W. Hall, UF/IFAS Department of Entomology and Nematology; b) William Barichivich, U.S. Geological Survey
All United States swallowtail larvae have eversible horn-like organs behind the head known as osmeteria. The osmeteria of spicebush swallowtail larvae are bright yellow in all larval instars. When threatened, larvae rear up, extrude the osmeterium, and attempt to smear the potential predator with a chemical repellent (Figure 24). The chemical makeup of the osmeterial secretion changes as the larvae mature. Fourth instars secrete terpenoids while the secretions of fifth instars are composed primarily of isobutyric and 2-methylbutyric acids (Ômura et al. 2006). It was suggested that the shift in chemicals secreted may reflect a change in response to different predator threats as the larvae mature. Eisner et al. (2005) suggested the possibility that even the secretions of the two prongs of the same osmeterium may differ. Both the terpenoids (Young et al. 1986) and butyric acids (Seligman and Doy 1973) of papilionid osmeterial secretions are reported to be synthesized in the osmeteria rather than sequestered from host plants.

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
The osmeterial repellent is effective against ants (Eisner and Meinwald 1965), but Berenbaum et al. (1992) reported that soldier bugs (Hemiptera: Pentatomidae) could attack and eat swallowtail larvae without evoking extrusion of the osmeteria.
The yellow prepupae are cryptic against the color of the leaf litter as they wander in search of pupation sites (Figure 25).

Credit: Donald W. Hall, UF/IFAS Department of Entomology and Nematology
Adults are Batesian (palatable) mimics of the poisonous and distasteful pipevine swallowtail, Battus philenor (L.) (Brower 1958).
Threatened Status
The spicebush swallowtail is threatened throughout its range due to mortality of its caterpillar host plants from laurel wilt fungus (Raffaelea lauricola T.C. Harr., Fraedrich and Aghayeva), which is transmitted by the introduced red bay ambrosia beetle (Xyleborus glabratus [Eichhoff]) (Coleoptera: Curculionidae: Scolytinae). The red bay ambrosia beetle may even be able to survive the harsh winters of the northern range of the spicebush swallowtail (Riggins and Formby 2015). The list of Lauraceae infected by the fungus (Smith 2015) includes all of the known hosts of the spicebush swallowtail. Only the exotic camphortree, Cinnamomum camphora, has shown any resistance at all to laurel wilt (Chupp and Battaglia 2014). Although survival of Cinnamomum camphora may support increased spicebush swallowtail populations (Gezon et al. 2019), it is classified as invasive by the University of Florida Institute of Food and Agricultural Sciences, and planting is not recommended.
Acknowledgements
The authors would like to acknowledge Marc Minno for reviewing this article and offering helpful suggestions. The authors also express appreciation to William Barichivich for permission to use his photograph of the squirrel tree frog (Figure 23).
Selected References
Arnaud PH. 1978. A Host-Parasite Catalog of North American Tachinidae (Diptera). United States Department of Agriculture Miscellaneous Publication 1319. Washington, D.C.
Berenbaum MR, Moreno B, Green E. 1992. Soldier bug predation on swallowtail caterpillars (Lepidoptera: Papilionidae): Circumvention of defensive chemistry. Journal of Insect Behavior 5: 547-553.
Borror DJ. 1960. Dictionary of Word Roots and Combining Forms: Compiled from the Greek, Latin, and other Languages, with Special Reference to Biological and Scientific Names. Mayfield Publishing Company. Palo Alto, California. 134 pp.
Brower JV. 1958. Experimental studies of mimicry in some North American butterflies. 2. Battus philenor and Papilio troilus, P. polyxenes and P. glaucus. Evolution 12: 123-136.
Carter M, Feeny P. 1999. Host-plant chemistry influences oviposition choice of the spicebush swallowtail butterfly. Journal of Chemical Ecology 25: 1999-2009.
Carter M, Feeny P, Haribal M. 1999. An oviposition stimulant for spicebush swallowtail butterfly, Papilio troilus, from the leaves of Sassafras albidum. Journal of Chemical Ecology 25: 1233-1245.
Cech R, Tudor G. 2005. Butterflies of the East Coast. Princeton University Press. Princeton, New Jersey. 345 pp.
Chupp AD, Battaglia LL. 2014. Potential for host shifting in Papilio palamedes following invasion of laurel wilt disease. Biological Invasions 16: 2639-2651.
Deering MD, Haslitt T, Scriber JM. 2003. Temperature determines diapause termination in Papilio troilus (Lepidoptera: Papilionidae). Holarctic Lepidoptera 10: 43-47.
Eisner T, Eisner M, Siegler M. 2005. Chap. 64. Class Insecta, Order Lepidoptera, Family Papilionidae, Eurytides marcellus, the zebra swallowtail butterfly, pp. 297-303. In Secret Weapons: Defenses of Insects, Spiders, Scorpions, and Other Many-legged Creatures. Harvard University Press. Cambridge, Massachusetts. 372 pp.
Eisner T, Meinwald YC. 1965. Defensive secretion of a caterpillar (Papilio). Science 150: 1733-1735.
Gatrelle RR. 2000. A new North American swallowtail butterfly: Description of a relict subspecies of Pterourus troilus (Papilionidae) from the southern tip of Florida. Taxonomic Report of the International Lepidoptera Survey 2: 1-13.
Gezon Z, Braatz EY, Duxbury C, Savage A, Daniels JC. 2019. Long-term trends in Persea palustris and Lauraceae-dependent butterfly species in central Florida before and after the introduction of laurel wilt disease. Journal of Insect Conservation 23: 341-350.
Glassberg J, Minno MC, Calhoun JV. 2000. Butterflies Through Binoculars: A Field, Finding, and Gardening Guide to Butterflies in Florida. Oxford University Press. New York. 242 pp.
Haddad NM, Hicks WM. 2000. Host pubescence and the behavior and performance of the butterfly Papilio troilus (Lepidoptera: Papilionidae). Environmental Entomology 29: 299-303.
Hagen RH, Scriber JM. 1991. Systematics of the Papilio glaucus and P. troilus species groups (Lepidoptera: Papilionidae): Inferences from allozymes. Annals of the Entomological Society of America 84: 380-395.
Hancock DL. 1983. Classification of the Papilionidae (Lepidoptera): A phylogenetic approach. Smithersia 2: 1-48.
Hazel WN. 1995. The causes and evolution of phenotypic plasticity in pupal color in swallowtail butterflies, pp. 205-210. In Scriber JM, Tsubake Y, Lederhouse RC. eds. Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publishers, Inc. Gainesville, Florida. 459 pp.
Howe WH. 1975. The Butterflies of North America. Doubleday. Garden City, NJ. 633 pp.
Imms A.D. 1957. A General Textbook of Entomology: Including the Anatomy, Physiology, Development and Classification of Insects. p. 212. Ninth Edition (entirely revised by Richards OW, Davies RG). Methuen. London. 886 pp.
Krombein KV, Hurd PD Jr, Smith DR, Burks BD. 1979. Catalog of Hymenoptera in America North of Mexico. Volume 1. Symphyta and Apocrita (Parasitica). Smithsonian Institution Press. Washington, D.C. 1198 pp.
Lederhouse RC. 1990. Avoiding the hunt: Primary defenses of lepidopteran caterpillars. pp. 175-189. In Evans DL, Schmidt JO. eds. Insect Defenses: Adaptive Mechanisms and Strategies of Prey and Predators. State University of New York Press. Albany, New York. 482 pp.
Lederhouse RC. 1995. Comparative mating behavior and sexual selection in North American swallowtail butterflies, pp. 117-131. In Scriber JM, Tsubake Y, Lederhouse RC. eds. Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publishers, Inc. Gainesville, Florida. 459 pp.
Lederhouse RC, Ayres MP, Nitao JK, Scriber JM. 1992. Differential use of lauraceous hosts by swallowtail butterflies, Papilio troilus and P. palamedes (Papilionidae). OIKOS 63: 244-252.
Mellencamp K, Hass M, Werne A, Stark R, Hazel W. 2007. Role of larval stemmata in control of pupal color and pupation site preference in swallowtail butterflies Papilio troilus, Papilio polyxenes, Eurytides marcellus, and Papilio glaucus (Lepidoptera: Papilionidae). Annals of the Entomological Society of America 100: 53-58.
Miller JS. 1987. Phylogenetic studies in the Papilioninae (Lepidoptera: Papilionidae). Bulletin of the American Museum of Natural History 186(4): 365-512.
Minno MC, Butler JF, Hall DW. 2005. Florida Butterfly Caterpillars and their Host Plants. University Press of Florida. Gainesville, Florida. 341 pp.
Minno MC, Minno M. 1999. Florida Butterfly Gardening. University Press of Florida. Gainesville, Florida. 210 pp.
Morris MW. 1989. Papilio troilus L. on a new and rare larval food plant. Journal of the Lepidopterists' Society 43: 147.
Nitao JK. 1995. Evolutionary stability of swallowtail adaptations to plant toxins. pp. 39-52. In Scriber JM, Tsubake Y, Lederhouse RC. eds. Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publishers, Inc. Gainesville, Florida. 459 pp.
Nitao JK, Ayres MP, Lederhouse RC, Scriber JM. 1991. Larval adaptation to lauraceous hosts: Geographic divergence in the spicebush swallowtail butterfly. Ecology 72: 1428-1435.
Ômura H, Honda K, Feeny P. 2006. From terpenoids to aliphatic acids: Further evidence for late-instar switch in osmeterial defense as a characteristic trait of swallowtail butterflies in the tribe Papilonini. Journal of Chemical Ecology 32: 1999-2012.
Opler PA, Krizek GO. 1984. Butterflies East of the Great Plains. The Johns Hopkins University Press. Baltimore, MD.
Opler PA, Malikul V. 1998. A Field Guide to Eastern Butterflies. Peterson Field Guides. Houghton Mifflin Company. New York.
Riggins J, Formby JP. 2015. Laurel wilt disease: Invasion potential and ecosystem impacts. Conference on Laurel Wilt Disease and Natural Ecosystems: Impacts, Mitigation and the Future. Coral Springs, Florida. UF/IFAS. Gainesville Florida.
Robinson GS, Ackery PR, Kitching IJ, Beccaloni GW, Hernández LM. Undated. HOSTS—a Database of the World's Lepidopteran Hostplants.
Scott JA. 1986. The Butterflies of North America. Stanford University Press. Stanford, California. 583 pp.
Scriber JM. 1996. Tiger tales: Natural history of native North American swallowtails. American Entomologist 42: 19-32.
Scriber JM, Deering MD, Francke LN, Wehling WF, Lederhouse RC. 1998. Notes on swallowtail population dynamics of three Papilio species in south-central Florida (Lepidoptera: Papilionidae). Holarctic Lepidoptera 5: 53-62.
Scriber JM, Lederhouse RC, Hagen RH. 1991. Foodplants and evolution within Papilio glaucus and Papilio troilus species groups (Lepidoptera: Papilionidae), pp. 341-373. In Price PW, Lewinsohn TM, Fernandes GW, Benson WW eds. Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. John Wiley. New York. 639 pp.
Seligman IM, Doy FA. 1973. Biosynthesis of defensive secretions in Papilio aegeus. Insect Biochemistry 3(10): 205-215.
Smith J. 2015. Welcome and introduction to laurel wilt. Conference on Laurel Wilt Disease and Natural Ecosystems: Impacts, Mitigation and the Future. Coral Springs, Florida. University of Florida Institute of Food & Agricultural Science. Gainesville, Florida.
Sperling FAH. 1993. Mitochondrial DNA variation and Haldanes's rule in the Papilio glaucus and P. troilus species groups. Heredity 71: 227-233.
Tyler HA. 1975. The Swallowtail Butterflies of North America. Naturegraph Publishers. Healdsburg, CA. 192 pp.
Tyler HA, Brown KS Jr., Wilson KH. 1994. Swallowtail Butterflies of the Americas. Scientific Publishers. Gainesville, FL. 376 pp.
Valella P, Scriber JM. 2003. Latitudinal variation in photoperiodic induction of pupal diapause in the spicebush swallowtail butterfly, Papilio troilus (Lepidoptera: Papilionidae). Holarctic Lepidoptera 10: 37-41.
Wagner DL. 2005. Caterpillars of Eastern North America. Princeton University Press. Princeton, New Jersey. 512 pp.
Warren AD, Davis K, Stangeland M, Pelham JP, Grishin NV. Undated. Butterflies of America. https://www.butterfliesofamerica.com/L/Papilionidae.htm
West DA, Hazel WN. 1985. Pupal color dimorphism in swallowtail butterflies—timing of the sensitive period and environmental control. Physiological Entomology 10: 1113-1119.
West DA, Hazel WN. 1996. Natural pupation sites of three North American swallowtail butterflies: Eurytides marcellus (Cramer), Papilio cresphontes Cramer, and P. troilus L. (Papilionidae). Journal of the Lepidopterists' Society 50: 297-312.
Young AM, Blum MS, Fales HM, Bian Z. 1986. Natural history and ecological chemistry of the neotropical butterfly Papilio anchisiades (Papilionidae). Journal of the Lepidopterists' Society 40(1): 36-53.