Introduction
The melaleuca gall midge, Lophodiplosis trifida Gagné, is a natural enemy of the invasive plant melaleuca (Australian broadleaved paperbark), Melaleuca quinquenervia (Cav.) S.T. Blake (Myrtales: Myrtaceae) in Florida (Figure 1). This species' ability to form galls on and damage melaleuca trees led to its study as a possible biological control agent and its eventual release in the United States.

Credit: Matthew Purcell, USDA and CSIRO
Distribution
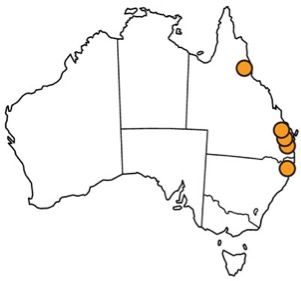
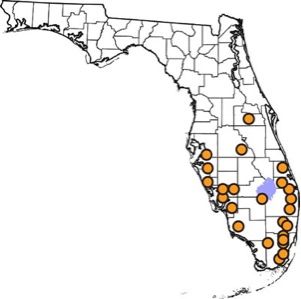
The melaleuca gall midge is native to eastern coastal areas of Queensland and New South Wales in Australia (Gagné et al. 1997; Gagné 2010). Specifically, the melaleuca gall midge has been collected near the towns of Tully and Woodburn, Roy's Road, and the Brisbane suburb of Indooroopilly (Gagné et al. 1997; Goolsby et al. 2002; Gagné 2010; Wineriter Wright and Center 2008) (Figure 2). In 2008, the melaleuca gall midge was approved for field release into Florida. It was subsequently released at 24 sites across southern Florida in 13 counties (Broward, Charlotte, Collier, Hendry, Hillsborough, Lee, Martin, Miami-Dade, Orange, Palm Beach, Polk, Sarasota, and St. Lucie) (USDA 2008; Pratt et al. 2013) (Figure 3). The released melaleuca gall midges survived at all release locations except one, due to a freeze at the site (Pratt et al. 2013). This locality was recolonized by melaleuca gall midges from nearby areas and the midge has subsequently spread to other areas in Florida where Melaleuca quinquenervia occurs (Pratt et al. 2013). The melaleuca gall midge has spread from the initial release sites at an average of 6.0 km/year (Pratt et al. 2013).
Credit: Matthew R. Moore, UF/IFAS
Credit: Matthew R. Moore, UF/IFAS
Description and Identification
Larvae
The three larval instars of the melaleuca gall midge feed, grow, and molt within prominent galls formed on young shoots of Melaleuca spp. (Gagné et al. 2009). The larvae of this midge are cylindrical, spindleform, and very small (Gagné et al. 2009). Larvae range in total length from 0.3–0.5 mm (first instar), 0.6–0.9 mm (second instar), and 1.3–2.0 mm (third instar) (Gagné et al. 2009). Melaleuca gall midge larvae can be distinguished from the other species of Lophodiplosis by the absence of setae (hairs) on most of the papillae (small body protuberances) and a unique three-toothed spatula on the prothorax of the third instar (Gagné et al. 2009) (Figure 4).
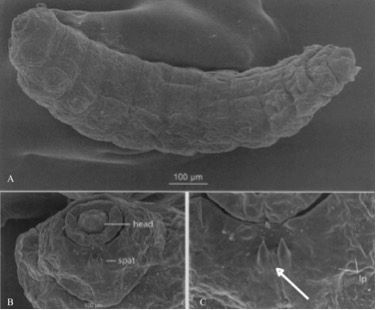
Credit: Recreated from figures 5, 6, and 7 in Gagné et al. (2009)
Pupae
Melaleuca gall midges pupate within their galls (Gagné 1997; Gagné et al. 2009). The pupae of Lophodiplosis species have specialized projections on the vertex of the head, evidently used to cut through plant gall tissue when the adults emerge (Gagné et al. 2009). The pupal vertex projection of the melaleuca gall midge is angled along its length and has three points at its apex (Gagné 1997).
Adults
Melaleuca gall midge adults are very small, 2.0–2.3 mm in length (Gagné 1997) (Figure 5). The females are slightly larger than males and have red or orange abdomens (filled with eggs) (Goolsby et al. 2002). Males have thinner abdomens that end in two distinctive hook-like cerci (Gagné 1997; Goolsby et al. 2002). Adult melaleuca gall midges can be distinguished from the other species in the genus Lophodiplosis by features of the male and female terminal abdominal segment (Gagné 1997).
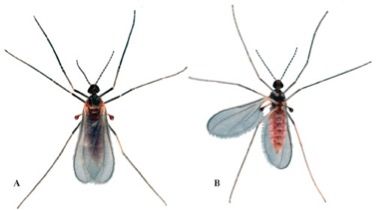
Credit: Lyle J. Buss, UF/IFAS
Life Cycle and Biology
The melaleuca gall midge was originally thought to live as an inquiline (i.e., present in the galls of other Lophodiplosis species) (Gagné 1997). Subsequent observations revealed that melaleuca gall midge forms galls on new stems of Melaleuca quinquenervia following flowering (Purcell et al. 2007). Persistent galls formed by melaleuca gall midges on Melaleuca quinquenervia result in damaged and deformed branches, plant dieback, blockage of vascular tissues, and sometimes plant death (Goolsby et al. 2002; Purcell et al. 2007) (Figure 6).

Credit: Paul D. Pratt, USDA
Melaleuca gall midge adults live only one to five days (Goolsby et al. 2002; USDA 2008). Females can lay hundreds of eggs on young leaves and stems during their short lives, with the average female laying 162 eggs under greenhouse conditions (Goolsby et al. 2002; USDA 2008). Eggs typically hatch after six days (USDA 2008).
Larvae hatch and burrow into plant stems where their salivary enzymes initiate gall formation in their Melaleuca hosts (Goolsby et al. 2002; USDA 2008). The galling is much more intense on lower branches of the tree (0–2 m high, > 75% of available branches are galled) and rates of galling decrease higher up the tree (Pratt et al. 2014). Galls formed by melaleuca gall midges can be monothalamous (having one chamber) or polythalamous (having multiple chambers) (USDA 2008). It takes about six weeks from the time that the larva emerges from the egg until the midge becomes an adult (Goolsby et al. 2002; USDA 2008) (Figures 7 and 8).

Credit: Paul D. Pratt, USDA

Credit: Lyle J. Buss, UF/IFAS
The host range of Lophodiplosis trifida is narrow (Goolsby et al. 2002; Wineriter Wright and Center 2008). Experiments demonstrated that melaleuca gall midges will oviposit on several Melaleuca species and at least one related Myrtaceae genus (Callistemon) (Goolsby et al. 2002; USDA 2008; Wineriter Wright and Center 2008). However, melaleuca gall midges complete their development only on Melaleuca quinquenervia and two other closely related Melaleuca species (Goolsby et al. 2002; USDA 2008; Wineriter Wright and Center 2008).
Biological Control of Melaleuca quinquenervia
Melaleuca is an invasive tree species in Florida wetland ecosystems, where it has the ability to form dense monocultures and greatly reduce plant species diversity. Melaleuca quinquenervia is native to Australia, New Guinea, and New Caledonia and was intentionally introduced into Florida multiple times dating back to the 1880s through 1959 (Craven and Lepschi 1999; Dray et al. 2006).
Melaleuca quinquenervia was and still is cultivated in various locations around the world including South America, Africa, Asia, Oceania, and the West Indies (Dray et al. 2006). This tree is valued as an ornamental plant due to its exotic-looking bark and flowers (Dray et al. 2006). Additionally, its bark and wood are used for packing material and lumber and the oils present in the leaves are used medicinally (Dray et al. 2006).
It is difficult to determine when melaleuca expanded into natural areas in Florida due to the lag time between when the tree escaped cultivation and became sufficiently dense to be noticed (Dray et al. 2006). By the 1920s, melaleuca had naturalized in cypress swamps in southwestern and southeastern Florida (Nehrling 1933; Small 1933). Surveys estimated that melaleuca occupies at least 200,000 ha of wetlands in Florida (Bodle et al. 1994; Laroche 1998).
Management of melaleuca is challenging due to its fire tolerance and ability to sprout again from stumps that have been cut (Center et al. 2008). This tree threatens several habitats in Florida including prairies, cypress swamps, pine forest, hammock, salt marshes, and mangroves (Dray et al. 2006; USDA 2008).
Introduction of the melaleuca gall midge into Florida was part of a larger control strategy for the melaleuca trees. Three different insect biological control agents of melaleuca have been released into Florida and have established. The leaf feeding weevil, Oxyops vitiosa (Pascoe) (Coleoptera: Curculionidae), was released in Florida in 1997 (Center et al. 2000, 2012). The weevil reduces the reproductive potential of melaleuca by consuming young foliage and destroying stem tips where flowers and fruits are produced (Balciunas et al. 1994; Center et al. 2000, 2012). The psyllid, Boreioglycaspis melaleucae Moore (Hemiptera: Psyllidae), released into Florida in 2002, was the second insect biocontrol agent of melaleuca (Center et al. 2006). The psyllid feeds on the juices of melaleuca and completes its entire life cycle on the plant (Purcell et al. 1997; Center et al. 2012).
In 2008, the third established biological control agent, the melaleuca gall midge, was released in Florida. Melaleuca gall midges are unlikely to kill or significantly damage mature melaleuca trees (USDA 2008). However, melaleuca gall midge damage can kill melaleuca seedlings and saplings, divert the tree's resources from growth and reproduction, and enhance the effects of other biological, chemical, and mechanical controls (USDA 2008; Tipping et al. 2008; Rodgers et al. 2014; Rodgers 2016). The melaleuca gall midge has maintained an extremely narrow host range in Florida because it completes development only on Melaleuca quinquenervia (Pratt et al. 2013).
A combination of control tactics (intensive monitoring, mechanical removal of trees, and biological control) has been successful at removing large monocultures of melaleuca and limiting regrowth in some parts of the Florida Everglades (Figure 9).

Credit: Reproduced from Rodgers (2016)
Selected References
Balciunas JK, Burrows DW, Purcell MF. 1994. Field and laboratory host ranges of the Australian weevil Oxyops vitiosa (Coleoptera: Curculionidae), a potential biological control agent for the paperbark tree, Melaleuca quinquenervia. Biological Control 4: 351–360. (30 September 2019)
Bodle MJ, Ferriter AP, Thayer DD. 1994. The biology, distribution, and ecological consequences of Melaleuca quinquenervia in the Everglades. p. 341-355. In Davis SM and Ogden JC (eds.), Everglades: The ecosystem and its restoration. St. Lucie Press. Delray Beach, Florida, United States. 860 p.
Center TD, Van TK, Rayachhetry M, Buckingham GR, Dray Jr. FA, Wineriter SA, Purcell MF, Pratt PD. 2000. Field colonization of the Melaleuca Snout Beetle (Oxyops vitiosa) in South Florida. Biological Control 19: 112–123. (30 September 2019)
Center TD, Pratt PD, Tipping PW, Rayamajhi MB, Van TK, Wineriter SA, Dray Jr. FA, Purcell MF. 2006. Field colonization, population growth, and dispersal of Boreioglycaspis melalucae Moore, a biological agent of the invasive tree Melaleuca quinquenervia (Cav.) Blake. Biological Control 39: 363–374. (30 September 2019)
Center TD, Pratt PD, Tipping PW, Rayamajhi, Wineriter SA, Purcell MF. 2008. Biological control of Melaleuca quinquenervia: Goal-based assessment of success. p. 657–666. In Julien M, Sforza R, Bon MC, Evans HC, Hatcher PE, Rector BG (eds.), Proceedings of the XII International Symposium on Biological Control of Weeds. CSIRO European Laboratory. Montpellier, France. 768 p. (30 September 2019)
Center TD, Purcell MF, Pratt PD, Rayamajhi MB, Tipping PW, Wright SA, Dray Jr. FA. 2012. Biological control of Melaleuca quinquenervia: an Everglades invader. BioControl 57: 151–165. (30 September 2019)
Craven LA, Lepschi BJ. 1999. Enumeration of the species and infraspecific taxa of Melaleuca (Myrtaceae) occurring in Australia and Tasmania. Australian Systematic Botany 12: 819–927. (10 February 2023)
Dray Jr. FA, Bennett BC, Center TD. 2006. Invasion history of Melaleuca quinquenervia (Cav.) S.T. Blake in Florida. Castanea 71: 210–225. (30 September 2019)
Gagné RJ. 2010. Update for a catalog of the Cecidomyiidae (Diptera) of the world. Systematic Entomology Laboratory, Agricultural Research Service, U. S. Department of Agriculture. Washington, D. C., United States. 544 p. (30 September 2019)
Gagné RJ, Balciunas JK, Burrows DW. 1997. Six new species of gall midges (Diptera: Cecidomyiidae) from Melaleuca (Myrtaceae) in Australia. Proceedings of the Entomological Society of Washington 99: 312–334. (30 September 2019)
Gagné RJ, Wright SA, Purcell MF, Brown BT, Pratt PD, Center TD. 2009. Description of the larva of Lophodiplosis trifida, an Australian gall midge (Diptera: Cecidomyiidae) and biocontrol agent of paperbark in Florida, USA. The Florida Entomologist 92: 593–597. (30 September 2019)
Goolsby J, Purcell M, Wright T, Makinson J, Zonneveld R, Brown B. 2002. 2002 Annual Report. Australian Biological Control Laboratory. United States Department of Agriculture. Agricultural Research Service. Office of International Research Programs. Australian Biological Control Laboratory. Indooroopilly, Queensland, Australia. 100 p. (10 February 2023)
Laroche FB. 1998. Managing melaleuca (Melaleuca quinquenervia) in the Everglades. Weed Technology 12: 726–732. (30 September 2019)
Nehrling H. 1933. The plant world in Florida; from the published manuscripts of Dr. Henry Nehrling; collected and edited by Alfred and Elizabeth Kay. MacMillan Co. New York, New York, United States. 304 p.
Pratt PD, Rayamajhi MB, Tipping PW, Center TD, Wright SA, Purcell M. 2013. Establishment, population increase, spread, and ecological host range of Lophodiplosis trifida (Diptera: Cecidomyiidae), a biological control agent of the invasive tree Melaleuca quinquenervia (Myrtales: Myrtaceae). Environmental Entomology 42: 925–935. (30 September 2019)
Pratt PD, Rayamajhi MB, Brown B, Purcell MF, Center TD. 2014. Within-plant distribution of the Melaleuca quinquenervia biological control agent Lophodiplosis trifida. Biocontrol Science and Technology 24: 1073–1076. (30 September 2019)
Purcell M, Wineriter S, Brown B. 2007. Lophodiplosis trifida Gagné (Diptera: Cecidomyiidae), a stem-galling midge with potential as a biological agent of Melaleuca quinquenervia (Myrtaceae). Australian Entomologist 34: 123–125.
Rodgers L, Black D, Bodle M, Laroche F. 2014. Chapter 7: Status of nonindigenous species. 2014 South Florida Environmental Report 1: 1–53. (30 September 2019)
Rodgers L. 2016. Chapter 7: Status of nonindigenous species. 2016 South Florida Environmental Report 1: 1–51. (30 September 2019)
Small JK. 1933. Manual of the southeastern flora: being descriptions of the seed plants growing naturally in Florida, Alabama, Mississippi, eastern Louisiana, Tennessee, North Carolina, South Carolina and Georgia. New York, New York, United States. 1554 pp. (30 September 2019)
Tipping PW, Martin MR, Pratt PD, Center TD, Rayamajhi MB. 2008. Suppression of growth and reproduction of an exotic invasive tree by two introduced insects. Biological Control 44: 235–241. (30 September 2019)
Turner CE, Center TD, Burrows DW, Buckingham GR. 1998. Ecology and management of Melaleuca quinquenervia, an invader of wetlands in Florida, U. S. A. Wetlands Ecology and Management 5: 165–178. (30 September 2019)
United States Department of Agriculture (USDA). Animal and Plant Health Inspection Service (APHIS). 2008. Field release of the biological control agent Lophodiplosis trifida Gagné (Diptera: Cecidomyiidae) for the control of Melaleuca quinquenervia (Cav.) S.T. Blake (Myrtales: Myrtaceae) in the continental United States. Environmental Assessment April 15, 2008. 30 pp. (30 September 2019)
Wineriter Wright SA, Center RD. 2008. Nonselective oviposition by a fastidious insect: The laboratory host range of the melaleuca gall midge Lophodiplosis trifida (Diptera: Cecidomyiidae). Biocontrol Science and Technology 18: 793–807. (30 September 2019)