Introduction
The genus Phoneutria is represented by eight species of spiders that all occur naturally only in Central and South America: Phoneutria boliviensis (Pickard-Cambridge), Phoneutria fera Perty, Phoneutria reidyi (Pickard-Cambridge), Phoneutria nigriventer (Keyserling), Phoneutria keyserlingi (Pickard-Cambridge), Phoneutria pertyi (Pickard-Cambridge), Phoneutria eikstedtae Martins and Bertani, and Phoneutria bahaiensis Simo and Brescovit (Simo and Brescovit 2001, Vetter and Hillebrecht 2008). Collectively, this group is referred to by a number of common names that include armed spiders, Brazilian wandering spiders and banana spiders in English speaking countries. In Brazil, they are known as "aranha armadeira", which translates as armed spider (Martins and Bertani 2007).
Phoneutria are nocturnal hunters that actively forage and overcome prey with potent venom rather than relying on a web for prey capture. Members of the genus are among the most medically important spiders in the world (Vetter and Isbister 2008). Phoneutria species are large spiders that vigorously defend themselves when threatened. Their venom consists of a mixture of peptides and proteins that together function as a potent neurotoxin in mammals (Richardson et al. 2006). In most spiders, venom serves as a method of subduing prey. However, in Phoneutria, venom may have evolved to perform a defensive function against mammals (Vetter and Isbister 2008). Pharmacologically, their venom has been extensively studied and its components have potential medical and agricultural uses (Gomez et al. 2002, Martin-Moutot 2006). Phoneutria spiders have high medical significance because, in addition to having potent venom, some species occur at high densities in and around populated areas within their native range. Outside their native range, they occasionally are imported accidentally in produce shipments originating from Central and South America (Vetter and Hillebrecht 2008, Vetter et al. 2014).
Synonymy
The genus Phoneutria was originally described by Perty in 1833 and included two species. During the subsequent century, various authors moved the Phoneutria species between the genera Phoneutria and Ctenus. In 1936, Phoneutria was restored by Mello-Leitao (Simo and Brescovit 2009); Phoneutria currently contains eight species (Vetter and Hillebrecht 2008).
Distribution
Phoneutria is a Neotropical (tropics of the Western Hemisphere) genus, occupying much of northern South America, with one species, Phoneutria boliviensis extending into Central America. There are records of Phoneutria species from Brazil, Colombia, Ecuador, Peru, Surinam, Guyana, northern Argentina, Uruguay, Paraguay, Bolivia, Mexico, Panama, Guatemala, and Costa Rica (Simo and Brescovit 2001, Vetter and Hillebrecht 2008). Within the genus, Phoneutria boliviensis is the most widespread, with a geographic range that extends from Central America south to Argentina. Phoneutria bahaiensis has the most restricted geographic distribution and occurs only in the Atlantic forests of the Brazilian states of Bahia and Espirito Santo. This species is considered precinctive to (only found in) Brazil and because of its narrow distribution, is listed on the Brazilian Environmental Ministry's Red List of threatened species (Dias et al. 2011).
Description
Phoneutria species are large and robust spiders in the family Ctenidae that superficially resemble large wolf spiders. The body length of these spiders ranges from 17–48 mm, while the leg span can reach 180 mm (Martins and Bertani 2007). The overall dorsal color of the body and legs is light brown, brown or grey (Figures 1 and 2). In some species, there are two longitudinal lines of lightly colored spots on the abdomen (Simo and Brescovit 2001). Within a species, the abdominal coloration and pattern can vary and is a poor character for differentiating the species.

Credit: Lawrence Reeves, UF/IFAS

Credit: Lawrence Reeves, UF/IFAS
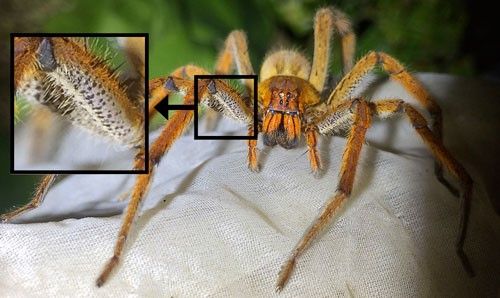
The body and legs of Phoneutria species are covered in short brown to grayish hairs (Lucas 1988). Many species (Phoneutria boliviensis, Phoneutria fera, Phoneutria keyserlingi, and Phoneutria nigriventer) have bright red hairs on their chelicerae (structures on the face, immediately above the fangs, Figure 3) and apparent bands of black and yellow or white on the underside of the two front pairs of legs (Figure 4). However, coloration is not a useful character for distinguishing between the species. Keys to the species of Phoneutria are available in Simo and Brescovit (2001) and Martins and Bertani (2007) and should be referred to if specific identifications are necessary (Vetter and Hillebrecht 2008).

Credit: Lawrence Reeves, UF/IFAS

Credit: Lawrence Reeves, UF/IFAS
The presence of dense scopulae (brushes of hair) on the pedipalps (leg-like appendages alongside the mouth) and an elaborate threat display are diagnostic characteristics of the genus (Lucas 1988, Martins and Bertani 2007). Phoneutria species resemble spiders in the genus Cupiennius Simon. Like Phoneutria, Cupiennius is a member of the Ctenidae family, but is largely harmless to humans. Because both genera have been detected in shipments of produce or cargo outside of their native ranges, it is important to distinguish between the two. Cupiennius species are routinely imported by accident to North America and Europe.
Spiders in this genus are detected in shipments to a substantially greater extent than Phoneutria species. Vetter et al. (2014) confirmed only seven accidental importations of Phoneutria species over an 80-year period, compared to 39 confirmed accidental importations of Cupiennius species over the same period. Figures 5 to 7 present two Cupiennius species, Cupiennius getazi Simon (Figure 5 and Figure 6) and Cupiennius coccineus, (Pickard-Cambridge) (Figure 7) which have been detected in cargo shipments outside of their native range. Cupiennius getazi can be identified by the presence of black dots on a white background on the underside of the two front pairs of legs (Figure 6). Cupiennius coccineus can be determined by the bright red underside of the two front pairs of legs. Refer to Vetter and Hillebrecht (2008) for a summary of additional morphological characters and geographical origins relevant to differentiating between these genera. Vetter et al. (2014) provides a key to the spider species that are most often detected in cargo and produce shipments outside of their native ranges. Ideally, identification of specimens discovered in imported produce or cargo and suspected to be a Phoneutria species should be determined by a specialist.

Credit: Lawrence Reeves, UF/IFAS
Credit: Harlan Gough, UF/IFAS

Credit: Lawrence Reeves, UF/IFAS
Life Cycle and Biology
Of the Phoneutria species, only the life cycle of Brazilian Phoneutria nigriventer is well known. The details of the Phoneutria life cycle may vary depending on species or geographic location. In Brazil, Phoneutria nigriventer males wander widely in search of females between March and May, corresponding to the time when most human envenomation incidents occur (Herzig et al. 2002). Bucherl (1969) reported that mating occurs in April and May. Subsequently, eggs are laid and deposited into sacs that are carried by the female. Female spiders may carry up to four egg sacs, collectively containing over 3,000 eggs. Immature Phoneutria nigriventer can capture prey immediately after leaving the egg sac. As the spiderlings grow, they must molt or shed their exoskeleton to allow for further growth.
In their first year, an immature spider will undergo between five and ten molts, depending on temperature and the amount of food consumed. As they mature, the frequency of molting decreases. In their second year, the growing spiders will molt three to seven times. During their third year, Phoneutria nigriventer molts only two to three times. Following one of these molts, the spiders typically become sexually mature. In captivity, Phoneutria nigriventer has a lifespan of up to six years (Bucherl 1969). As Phoneutria spiders mature, the proteins present in their venom change, becoming increasingly lethal to vertebrates (Herzig et al. 2004).
When confronted by a potential predator, all members of the genus exhibit a characteristic threat display (Martins and Bertani 2007) (Figure 8). Phoneutria spiders are more prone to holding their ground and utilizing this display than to retreating (Lucas 1988). The spider stands on the two posterior pairs of legs, with the body oriented nearly perpendicular to the ground. The two pairs of forelegs are thrust upwards and held above the body, displaying the brightly colored underside of the legs. The spider sways its legs laterally and shifts towards the movements of the threat, while displaying the fangs and bristling spines on the legs.

Credit: Lawrence Reeves, UF/IFAS
Phoneutria spiders are nocturnal hunters that do not construct webs to capture prey. They feed on other invertebrates and small vertebrates such as frogs (Pacheco et al. 2016, Foerster et al. 2017). Phoneutria boliviensis sometimes wraps captured prey in silk, affixing it to the substrate, usually a vertical perch (Hazzi 2014). During the day, Phoneutria spiders seek shelter within vegetation, crevices in trees or inside termite mounds. Species in the genus actively forage for arthropod and small vertebrate prey in the understory vegetation and on the ground. Some species often utilize large-leaved plants such as palms as a substrate for hunting. Torres-Sanchez and Gasnier (2010) hypothesize that doing so allows immature spiders to avoid larger spiders that are potential predators on the ground, while providing the ability to better sense the vibrations of an approaching predator.
Medical Importance
Worldwide, there are approximately 40,000 described spider species, most of which use venom to subdue prey. Of these, very few are medically important to people. Before 2000, spiders were estimated to be responsible for fewer than 200 deaths per year globally (Nentwig and Kuhn-Nentwig 2013). Per million people, spiders killed between 0.02 and 0.04 people per year. In comparison, snakes and scorpions respectively caused 20 and 0.1–1.4 fatalities per million people each year. The most medically important spiders include the widow spiders (Latrodectus, Theridiidae), the recluse spiders (Loxosceles, Sicariidae), the Australian funnel-web spiders (Atrax and Hadronyche, Hexathelidae) and armed spiders (Phoneutria, Ctenidae) (Vetter and Isbister 2008). In the past three decades, there have not been any confirmed fatalities due to envenomation by widow spiders, Australian funnel-web spiders or armed spiders (Nentwig and Kuhn-Nentwig 2013). Among the recluse spiders, bites are easily misdiagnosed, making it difficult to determine figures regarding their bites. Despite the low frequency of fatalities attributed to these taxa around the world, bites from species in these groups, including Phoneutria, can be serious and often require medical treatment.
As with other spiders of medical importance, venom is injected into prey or defensively into potential predators through the fangs (Figure 9). Venom is produced by glands located in the chelicerae (structures on the face, immediately above the fangs). The venom of Phoneutria spiders consists of a mixture of proteins and peptides that are active against the nervous systems of both invertebrates and vertebrates (Gomez et al. 2002). Among species in the genus, venom composition and potency vary, with Phoneutria nigriventer and Phoneutria keyserlingi having particularly potent venoms (Vetter and Hillebrecht 2008). While these and other Phoneutria species are primarily associated with forested habitats, Phoneutria nigriventer and Phoneutria keyserlingi can occupy habitats in rural and urban areas. Both species also are frequently found inside human dwellings, where they prey on cockroaches and other pest arthropods. As a result, bites from these and other Phoneutria species are common. For example, in 2006, 2,687 cases of envenomation were treated in Brazil alone (Bucaretchi et al. 2008).

Credit: Lawrence Reeves, UF/IFAS
Over the past 100 years, 10 fatalities have been attributed to Phoneutria spiders, mostly among young people (Nentwig and Kuhn-Nentwig 2013). In comparison, similar numbers of fatalities are reported for the widow spiders and Australian funnel-web spiders. While cases of mortality are known, in the majority of cases (90%), Phoneutria envenomation is considered to be mild and only 0.5–3.3% are diagnosed as severe or systemic (Bucaretchi et al. 2008). The effects of envenomation include severe pain, elevated heart rate, arterial hypertension, cardiac distress, shock, muscle tremors, priapism and frequent vomiting (Gomez et al. 2002). These symptoms can be particularly pronounced in children. In Brazil, moderate and severe cases (about 3% of cases) of envenomation are treated with anti-venom but are otherwise treated symptomatically (Bucaretchi et al. 2016).
Envenomation by Phoneutria spiders is a reasonable concern only within their native range. These species are common in forested habitats, but also will occupy populated and agricultural areas, bringing them into contact with humans. Accidents are particularly common in banana plantations, where the spiders often seek shelter in bunches of bananas during the day. This behavior enables their accidental importation into areas outside of their natural Neotropical distribution. Phoneutria species have been intercepted in Europe and North America (Vetter and Hillebrecht 2008, Vetter et al. 2014). Of the Phoneutria species, Phoneutria boliviensis is the most common spider intercepted in international shipments, in part because it is the species with the widest distribution. Many banana and other agricultural shipments originate in Central America, where this species occurs.
Compared to other Phoneutria species, the venom of Phoneutria boliviensis is less potent, and envenomations are typically mild (Vetter and Hillebrecht 2008). The Phoneutria species with the most potent venoms, Phoneutria nigriventer and Phoneutria keyserlingi, are not widely exported because they occur in Brazil, where much of the country's banana crop is consumed locally. In addition, most other Phoneutria species occur in regions of Brazil or the Amazon that are sparsely populated and produce few internationally traded products. Vetter and Hillebrecht (2008) also caution against the misidentification of Phoneutria with the essentially harmless genus, Cupiennius. Cupiennius share some morphological characters with Phoneutria, including a large body size and red hairs on the chelicerae (in some species). Like Phoneutria, they are common in agricultural settings, particularly banana plantations.
References
Bucaretchi F, Bertani R, De Capitani EM, Hyslop S. 2016. "Envenomation by wandering spiders (genus Phoneutria)." In: Gopalakrishnakone P. (editor). Clinical Toxinology, Springer, Berlin, Germany.
Bucaretchi F, Mello SM, Vieira RJ, Mamoni RL, Souza MH, Antunes E, Hyslop S. 2008. "Systematic envenomation caused by the wandering spider, Phoneutria nigriventer, with quantification of circulating venom." Clinical Toxicology 46: 885–889.
Bucherl W. 1969. "Biology and venoms of the most important South American spiders of the genera Phoneutria, Loxosceles, Lycosa, and Latrodectus." American Zoologist 9: 157–159.
Dias MA, Simo M, Castellano A, Brescovit AD. 2011. "Modeling distribution of Phoneutria bahaensis (Araneae, Ctenidae): An endemic and threatened spider from Brazil." Zoologia 28: 432–439.
Foerster NE, Carvalho BHG, Conte CE. 2017. "Predation on Hypsiboas bischoffi (Anura: Hylidae) by Phoneutria nigriventer (Araneae: Ctenidae) in southern Brazil." Herpetology Notes 10: 403–404.
Gomez MV, Kalopothakis E, Guatimosim C, Prado MAM. 2002. "Phoneutria nigriventer venom: A cocktail of toxins that affect ion channels." Cellular and Molecular Neurobiology 22: 579–588.
Hazzi NA. 2014. "Natural history of Phoneutria boliviensis (Araneae: Ctenidae): Habitats, reproductive behavior, postembryonic development and prey-wrapping." Journal of Arachnology 42: 303–310.
Herzig V, Ward RJ, dos Santos WF. 2002. "Intersexual variation in the venom of the Brazilian "armed" spider, Phoneutria nigriventer (Keyserling 1891)." Toxicon 40: 1399–1406.
Herzig V, Ward RJ, dos Santos WF. 2004. "Ontogenetic changes in Phoneutria nigriventer (Araneae, Ctenidae) spider venom." Toxicon 44: 635–640.
Lucas S. 1988. "Spiders in Brazil." Toxicon 26: 759–772.
Martin-Moutot N, de Haro L, dos Santos RG, Mori Y, Seagar M. 2006. "Phoneutria nigriventer small omega-phonetoxin IIA: A new tool for anti-calcium channel autoantibody assays in Lambert-Eaton myasthenic syndrome." Neurobiology of Disease 22: 57–63.
Martins R, Bertani R. 2007. "The non-Amazonian species of the Brazilian wandering spiders of the genus Phoneutria Perty, 1833 (Araneae, Ctenidae), with the description of new species." Zootaxa 1526: 1–36.
Nentwig W, Kuhn-Nentwig L. 2013. "Spider venoms potentially lethal to humans." p. 253–264. In Nentwig W (editor). Springer, Berlin, Germany: Spider Ecophysiology.
Pacheco EO, Ferreira VG, Pedro FMSR, Santana DJ. 2016. "Predation on Scinax crospedospilus (Anura: Hylidae) by Phoneutria nigriventer (Araneae: Ctenidae) in an Atlantic Forest fragment in southeastern Brazil." Herpetology Notes 9: 315–316.
Simo M, Brescovit AD. 2001. "Revision and cladistics analysis of the Neotropical spider genus Phoneutria Perty, 1833 (Araneae, Ctenidae), with notes on related Cteninae." Bulletin of the British Arachnological Society 12: 67–82.
Torres-Sanchez MP, Gasnier TR. 2010. "Patterns of abundance, habitat use and body size structure in Phoneutria reidyi and P. fera (Araneae: Ctenidae) in a central Amazonian forest." Journal of Arachnology 38: 433–440.
Vetter RS, Hillebrecht S. 2008. "Distinguishing two often-misidentified genera (Cupiennius, Phoneutria) (Araneae: Ctenidae) of large spiders found in Central and South American cargo shipments." American Entomologist 54: 88–92.
Vetter RS, Crawford RL, Buckle DJ. 2014. "Spiders (Araneae) found in bananas and other international cargo submitted to North American arachnologists for identification." Journal of Medical Entomology 51: 1136–1143.
Vetter RS, Isbister GK. 2008. "Medical aspects of spider bites." Annual Review of Entomology 53: 409–429.