The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The bumelia webworm moth, Urodus parvula (Edwards) is a small, smoky colored moth that is sometimes encountered in light trap collections. The caterpillars (Figure 1) occasionally occur in isolated outbreaks (Watson 1914, Johnson and Lyon 1988) during which they defoliate red bays, Persea borbonia (L.) Spreng.

Credit: Lyle J. Buss, UF/IFAS
Nomenclature and Classification
Edwards (1881) created the genus Penthetria in the family Zygaenidae and described two new species in the genus, Penthetria parvula and Penthetria majuscula (now Cydosia majuscula [Edwards]: Noctuidae). These species are similar in appearance and were often confused after Holland (1903, Pl. 29, Fig. 66) mistakenly illustrated parvula as majuscula (Kimball 1965). Bonniewell (1918) reported collecting many larvae and pupae of Cydosia majuscula in Florida, but the cocoons he described were likely those of parvula, not majuscula. In fact, it is unlikely that Cydosia majuscula is resident in Florida (Kimball 1965, Heppner 2007).
Dyar (1898) synonymized the genus Penthetria Edwards with the genus Cydosia Westwood (family Noctuidae). He then stated that the species parvula was not assigned to any genus at that time. Busck (1910) placed Penthetria parvula in the genus Trichostibas, and Dyar (1913) classified it in the family Yponomeutidae. Finally, Forbes (1923) moved Trichostibas parvula to the genus Urodus Herrich-Schäffer 1854 (Herrich-Schäffer 1850–1858).
For many years Urodus parvula continued to be included in the family Yponomeutidae (e.g., Covell 1984, Heppner 1987) until Kyrki (1988) made Urodus the type genus of the new family Urodidae.
The common name for urodids is false burnet moths (Heppner 1998, Beadle and Leckie 2012) because of the resemblance of some urodids to burnet moths in the family Zygaenidae.
Distribution
The bumelia webworm occurs primarily in the coastal plain of the southeastern US from the District of Columbia westward to eastern Texas and north to western Tennessee (Figure 2). There is also an isolated record from western Virginia (Moth Photographers Group [http://mothphotographersgroup.msstate.edu/species.php?hodges=2415]).
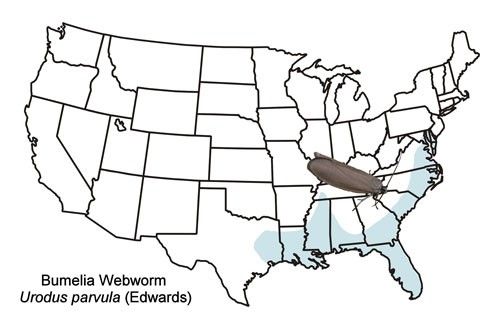
Credit: Donald W. Hall, UF/IFAS, (based primarily on records from the map)
Description
Adults
The wingspread is 2 to 2.9 cm (approx. 0.8 to 1.14 in) (Covell 1984). The forewings and body are smoky black, and the hindwings are lighter in color and somewhat translucent (Figure 3). Each forewing has an accessory cell (=chorda) (Heppner 1998) (Figure 4) that is difficult to see unless the scales are removed, or the wing is cleared. The antennae are filiform, and ocelli (simple eyes) and chaetosema (sensory setae on head near eyes) are lacking. These characteristics distinguish Urodus parvula adults from the similar adults of the laurelcherry smoky moth, Tarmann (Zygaenidae), which have bi-pectinate antennae and both ocelli and chaetosemata (Figure 5). The labial palpi of Urodus parvula are up-curved (Heppner 1998) while those of Neoprocris floridana are straight. Zygaenidae lack forewing accessory cells.

Credit: Lyle J. Buss, UF/IFAS

Credit: Donald W. Hall, UF/IFAS

Credit: Donald W. Hall, UF/IFAS
Males and females are similar in appearance. Males can be distinguished from females by their large external genitalia and by the short reddish area at the base of each wing near the costal margin (Frost 1972).
The typical resting position of urodids is with the wings folded back and roof-like over the abdomen (Figure 6).

Credit: Lyle J. Buss, UF/IFAS
Eggs
Eggs are approximately 0.74 mm by 0.37 mm (0.03 in by 0.07 in), and the chorion (eggshell) is pale yellow and unsculptured (Frost 1972).
Larvae
Mature larvae are approximately 12 mm (0.47 in) long (Frost 1972). The head is yellow. The thorax and abdomen have middorsal and lateral longitudinal rows of irregular white patches with yellowish-orange spots. Transverse rows of elevated black pinacula (areas of sclerotized cuticle) bearing stout black setae arise from the white patches. (Figure 7).

Credit: Lyle J. Buss, UF/IFAS
Cocoons and Pupae
The mesh cocoon is approximately 13 mm (0.51 in) long and is suspended from the substrate by a silk stalk. The larva leaves an opening at the rear (Figure 8) through which the larval exuviae is expelled (Forbes 1923) (Figure 9) and an anterior opening for pupal protrusion prior to adult emergence (Figure 10).

Credit: Lyle J. Buss, UF/IFAS

Credit: Lyle J. Buss, UF/IFAS

Credit: Donald W. Hall, UF/IFAS
The pupa has a cremaster composed of hooked setae, eight hooked setae on the head, and transverse rows of backward-directed spines on some abdominal segments (Figure 11). The hooked setae of the cremaster and those on the head probably anchor the pupa in the cocoon. The cremaster also anchors the pupal exoskeleton during adult eclosion. The transverse rows of spines have been observed in other moth species to function in protrusion of the pupa prior to adult eclosion.
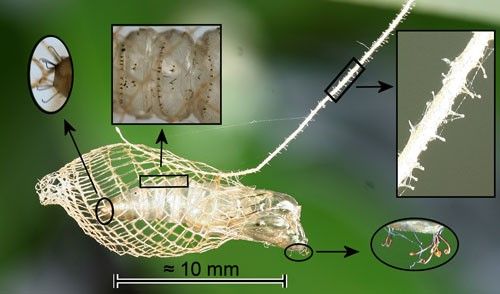
Credit: Donald W. Hall, UF/IFAS
Life Cycle
Urodus parvula adults are attracted to lights and are sometimes taken in light traps. Adults are active throughout much of the year in Florida and are sometimes abundant during the winter and spring at the Archbold Biological Research Station in central Florida (Frost 1972). Nothing is known of their mating behavior.
Watson (1914) stated that although adults are capable of short flights, they commonly crawl up the trunks of red bay hosts to lay their eggs.
As they feed, larvae cut characteristic notches in the edges of leaves (Figure 12). When they have defoliated a host plant or are mature, they drop to the ground on silk threads and wander to find other hosts or to pupate on surrounding objects (often houses, fences, or tree trunks) (Watson 1914). In an outbreak in 1913 in Seabreeze, Florida, larvae were so common in February and again in April on Persea sp. that pedestrians carried umbrellas to keep them off (Watson 1914).

Credit: Lyle J. Buss, UF/IFAS
Hosts
Urodus parvula is most common on red bay, Persea borbonia (L.) Sprengel (Figures 13 and 14).

Credit: Donald W. Hall, UF/IFAS

Credit: Donald W. Hall, UF/IFAS
Urodus parvula is known by the common name bumelia webworm because it has been recorded feeding on Florida bully, Sideroxylon reclinatum Michaux (synonym: Bumelia reclinata Vent.) (Frost 1972). Leaves of Sideroxylon reclinatum are variable in size and shape (elliptic to obovate) from plant to plant (Figures 15 and 16) or sometimes even on the same plant (Godfrey 1988). Urodus parvula is also recorded from a variety of other plants including oak, hibiscus, and orange (Frost 1972), avocado (Persea americana Mill.) and Camellia japonica L. (Heppner 2007). However, as larvae wander from a defoliated host plant or when they are ready to spin their cocoons, they may wander onto nearby non-host plants. Consequently, some of these host listings may not be valid.

Credit: Paul Corogin, Florida Museum of Natural History, University of Florida

Credit: Donald W. Hall, UF/IFAS
Natural Enemies
The only parasitoids reported from Urodus parvula are two tachinid flies:
- Hyphantrophaga boarmiae Coquillet (O'Hara 2013), formerly Zenillia boarmiae (Coquillet) (Patton 1958).
- Euceromasia floridensis Reinhard (Reinhard 1957)
Urodus parvula is not known to be chemically defended and is likely eaten by many invertebrate and vertebrate (e.g., birds and lizards) predators.
Defenses
Larva
Larvae thrash around vigorously when handled (Watson 1914) and may also do so when threatened by predators. Larvae of at least six other families of Lepidoptera, when disturbed, drop toward the ground on "lifelines" of silk thread (Sugiura and Yamazaki 2006) presumably to escape predators. Urodus parvula may also use this behavior to escape predation.
Urodus parvula larvae may gain protection from predators by their resemblance to Neoprocris floridana larvae which can be quite common, and which are chemically defended by cyanogenic glycosides.
Cocoon and Pupa
Eisner (2003, p.400) stated, "The cocoon, although loosely spun, doubtless protects the pupa against predation." but offered no hypothesis to explain the possible mechanism. Bates (1892) described a stalked, mesh cocoon from the area of Ega (now Tefé), Amazonas, Brazil. He stated that the threads of the cocoon were so stout that they were unlikely to be torn by the beaks of insectivorous birds, and that the pendulous stalk would cause the cocoon to give way when birds peck at it - making it doubly secure.
The loose mesh of the cocoon may provide ventilation to prevent mold in humid environments.
When the stalk of Trichostibas isthmiella (Busck) was disturbed, the pupa jerked violently which would likely shake off ants (Busck 1910) which are the most likely predators. The stalk of Urodus parvula (and probably those of other urodids) is covered with stalked knobs (Figure 11). These may serve to entangle small parasitoids or predators.
Pushing out the larval exuviae during pupation may minimize attraction of enemies to the cocoon by volatile chemicals emitted by the exuviae. Volatiles from exuviae can serve as attractants to parasitoids (Afsheen et al. 2008). Busck (1910) suggested that odors from the exuviae might attract predacious ants.
Acknowledgments
The author would like to acknowledge Andrei Sourakov and Howard Frank for reviewing this article and offering helpful suggestions and Paul Corogin for providing the photographs of Sideroxylon reclinatum for Figure 15.
Selected References
Afsheen S, Wang X, Li R, Zhu C-S, Lou Y-G. 2008. "Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products." Insect Science 15: 381–397.
Bates HW. 1892. The Naturalist on the River Amazons: A Record of the Adventures, Habits of Animals, Sketches of Brazilian and Indian Life, and Aspects of Nature under the Equator, during Eleven Years of Travel. London: John Murray.
Beadle D, and Leckie S. 2012. Peterson Field Guide to Moths. New York, NY: Houghton Mifflin Harcourt.
Bonniwell JG. 1918. "Notes on collecting in Florida." The Lepidopterist 2(8): 57–60.
Busck A. 1910. "New moths of the genus Trichostibas." Proceedings of the U.S. National Museum 38(1765): 527–530. +Plate 35.
Covell C. 1984. A Field Guide to the Moths of Eastern North America. Boston, MA: Houghton Mifflin.
Dyar HD. 1898. "New American moths and synonymical notes." Journal of the New York Entomological Society 6: 33–44.
Dyar HD. 1913. "The larva of Trichostibas parvula (Lepidoptera: Yponomeutidae)." Insecutor Inscitiae Menstruus 1(1): 48–49.
Edwards H. 1881. "A new genus and some new forms of North American Zygaenidae." Papilio 1: 80–81.
Eisner T. 2003. For Love of Insects. Cambridge, MA: Harvard University Press.
Forbes WMT. 1923. The Lepidoptera of New York and Neighboring States. Primitive Forms, Microlepidoptera, Pyralids, Bombyces. Memoir 68. Ithaca, NY: Cornell University Agricultural Experiment Station.
Frost SW. 1972. "Notes on Urodus parvula (Henry Edwards) (Yponomeutidae)." Journal of the Lepidopterists' Society 26(3): 173–177.
Godfrey RK. 1988. Trees, shrubs, and woody vines of northern Florida and adjacent Georgia and Alabama. Athens, GA: University of Georgia Press.
Heppner JB. 1987. "Yponomeutidae (Yponomeutoidea)." In: Stehr FW (ed.) Immature Insects. Dubuque, IA: Kendall/Hunt.
Heppner JB. 1998. "Classification of Lepidoptera." Holarctic Lepidoptera 5 (Supplement 1): 1–148.
Heppner JB. 2007. Lepidoptera of Florida. Part 1. Introduction and Catalog. Gainesville, FL: Florida Department of Agriculture and Consumer Services. Division of Plant Industry.
Herrich-Schäffer G. A. W., 1850–1858. Sammlung neuer oder wenig bekannter aussereuropäischer Schmetterlinge. Vol. 1. Regensburg, Germany. 84 pp., 96 + 24 plates.
Holland WJ. 1903. The Moth Book: A Guide to the Moths of North America. New York: Doubleday, Page.
Johnson WT, and Lyon HH. 1988. Insects that Feed on Trees and Shrubs. Ithaca, NY: Cornell University Press. 2nd ed.
Kimball CP. 1965. Lepidoptera of Florida. Arthropods of Florida and Neighboring Land Areas. Gainesville, FL: Florida Department of Agriculture and Consumer Services. Division of Plant Industry.
Kyrki J. 1988. "The systematic position of Wockia Heinemann, 1870, and related genera (Lepidoptera: Ditrysia: Yponomeutidae auct.)." Nota Lepidopterologica 11(1): 45–69.
O'Hara JE. 2013. "Genus Hyphantrophaga Townsend 1892." Taxonomic and host catalogue of the Tachinidae of America North of Mexico. Accessed March 8, 2018 http://www.nadsdiptera.org/Tach/Nearctic/CatNAmer/Genera/Hyphantrophaga.html
Patton CN. 1958. "A catalog of the Larvaevoridae of Florida." Florida Entomologist 41(2): 77–89.
Reinhard HJ. 1957. "New American muscoid Diptera (Sarcophagidae, Tachinidae)." Entomological News 68(4): 99–111.
Sugiura S, Yamazaki K. 2006. "The role of silk threads as lifelines for caterpillars: pattern and significance of lifelineclimbing behavior." Ecological Entomology 31: 52–57.
Watson JR. 1914. Trichostibas parvula. University of Florida. Florida Agricultural Experiment Station Annual Report for 1913. Report of Entomologist. Destructive Insects of the Year. pp. lxx-lxxi. E.O. Deland, FL: Painter Printing Company.