Introduction
Golf contributes to the quality of life of many residents and visitors to the state of Florida and generates billions of dollars for our state economy. Some of the criteria that are used to designate a good course are ball speed and evenness of the playing surface and green healthy grass. Each of these quality parameters can be negatively affected by plant-parasitic nematodes (Figure 1). Of all the pests that commonly affect golf course turf in Florida, nematodes are probably the least understood and most difficult to manage. Nematode problems are more common and more severe in Florida than in most other states because our mild climate and sandy soils provide a perfect habitat for many of the most destructive nematode species.
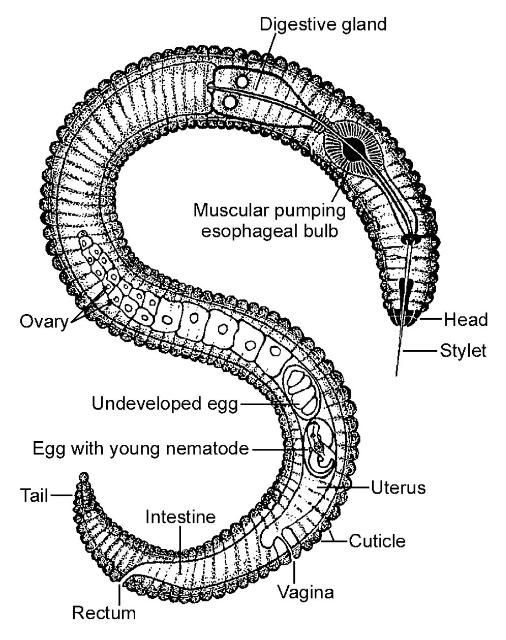
Credit: R. P. Esser, Florida Department of Agriculture and Consumer Services, Division of Plant Industry; used with permission.
Plant-Parasitic Nematodes
Nematodes are unsegmented roundworms. Not all nematodes are bad; in fact, most species are beneficial, feeding on bacteria, fungi, or other microscopic organisms. There are even nematodes that can be used as biological control organisms to help manage important turf insect pests. However, there also are genera of nematodes that are pests or pathogens of animals or plants. Nematodes that feed on plants are called plant-parasitic nematodes. Plant-parasitic nematodes are very small, and microscopes are required to see them (Figure 2). Plant-parasitic nematodes have a stylet or mouth-spear that is similar in structure and function to a hypodermic needle (Figure 3a, b). The stylet is used to puncture plant cells, and then the nematode can inject digestive juices and ingest plant fluids through it. Plant-parasitic nematodes are divided into groups based on how they feed on plants.
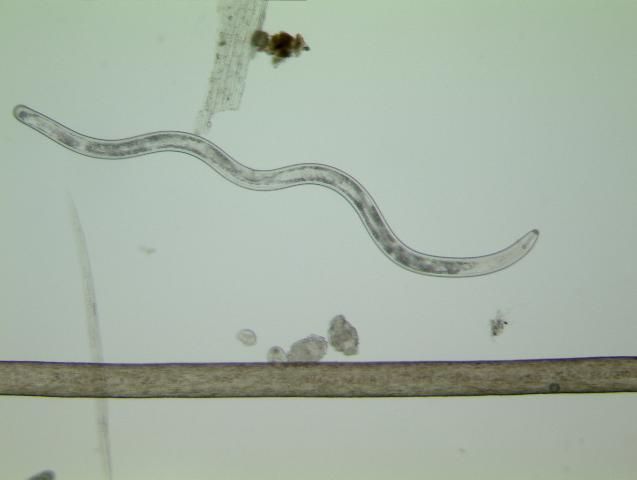
Credit: W. T. Crow, UF/IFAS Entomology and Nematology Department

Credit: J. E. Luc, UF/IFAS Entomology and Nematology Department

Credit: J. E. Luc, UF/IFAS Entomology and Nematology Department
Ectoparasites feed by inserting just their stylet into roots while their body remains outside in the soil (Figure 4). Because they spend their entire life in the soil, contact nematicides can work well for these nematodes. Some common genera of ectoparasitic nematodes that damage turf in Florida are sting nematode (Belonolaimus), stubby-root nematodes (Trichodorus and Nanidorus), spiral nematodes (Helicotylenchus), and ring nematodes (Mesocriconema).
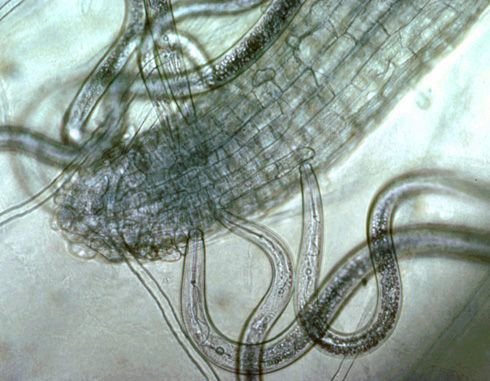
Credit: J. O. Becker, UC Riverside, used with permission.
Migratory endoparasites enter into plant tissue with their body and tunnel around, feeding as they move from cell to cell (Figure 5). As they tunnel through plant roots' they disrupt the vascular tissues and prevent roots from functioning properly. These nematodes typically lay their eggs within roots. Because the nematodes and their eggs are largely within roots, systemic nematicides usually work best against migratory endoparasitic nematodes. The most common genera of migratory endoparasites that affect turf in Florida are lance nematodes (Hoplolaimus).
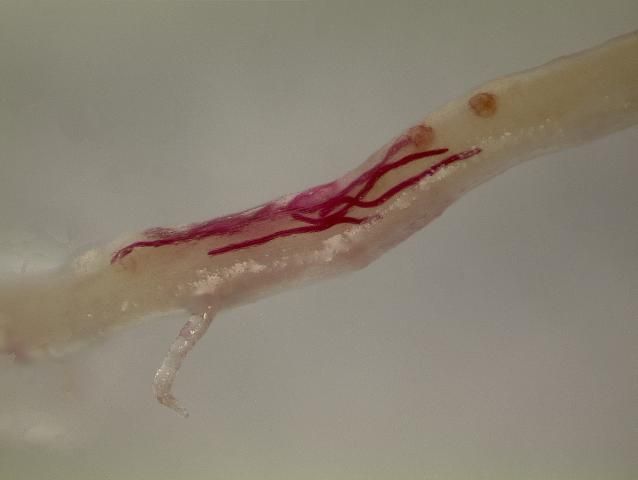
Credit: A. C. Hixson, UF/IFAS Entomology and Nematology Department
Sedentary endoparasites enter into roots and then inject hormones that cause specialized feeding sites to develop. After initiating a feeding site, these nematodes will no longer move and will lose their typical worm-like shape. Females become round or pear-shaped (Figure 6). They lay their eggs in masses that can contain several hundred eggs each. The egg masses may occur within the root tissue or be exposed at the root surface. While systemic nematicides typically are more effective for sedentary endoparasites, contact nematicides can affect eggs exposed at the root surface. The most common genera of sedentary endoparasites on turf in Florida are the root-knot nematodes (Meloidogyne).
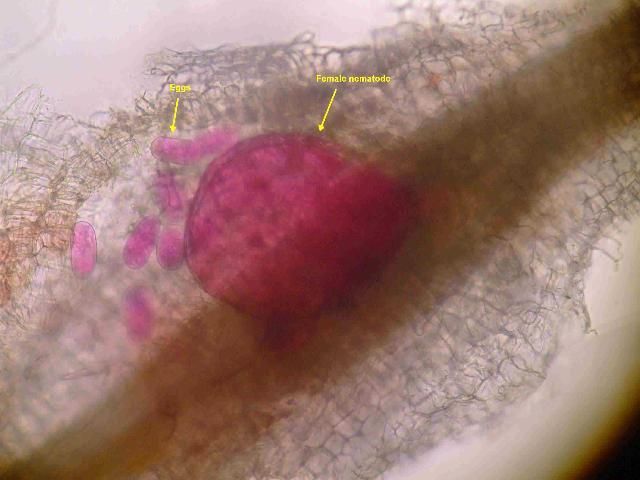
Credit: N. S. Sekora, UF/IFAS Entomology and Nematology Department
Nematodes' Effects on Turf
All of the plant-parasitic nematodes that damage turf in Florida feed on roots. As plant-parasitic nematodes feed, they damage plants' root systems and reduce their ability to obtain water and nutrients from the soil. This makes the turf more susceptible to drought and other stresses. When nematode population densities get high enough, or when environmental stresses occur, aboveground symptoms may become evident. Grass may wilt and die. Research has shown that the ability of nematode-damaged turf roots to get water and nutrients from soil is impaired. Nematode-damaged turf typically needs frequent irrigation to avoid wilting and decline. Also, the potential for nutrient leeching is higher from nematode-damaged turf.
Symptoms of a Nematode Problem
Aboveground symptoms of nematode damage include wilting, thinning, or death (Figure 7). Plant-parasitic nematodes usually occur in clumps, so nematode damage usually occurs in irregularly shaped patches that may enlarge slowly over time. Often, as the grass thins, weeds such as spurge may become prominent (Figure 8). This is because the nematode-damaged grass is less competitive with the weeds. Root-knot nematodes cause yellow blotches on golf greens that can appear similar to several fungal diseases (Figure 9).

Credit: W. T. Crow, UF/IFAS Entomology and Nematology Department

Credit: W. T. Crow, UF/IFAS Entomology and Nematology Department

Credit: W. T. Crow, UF/IFAS Entomology and Nematology Department
Belowground symptoms on turf roots vary depending on the types of nematodes present. Ectoparasites often cause roots to be short and stubby (Figure 10). Endoparasites often cause roots to be dark and rotten looking (Figure 11). Both ecto- and endo-parasites cause a reduction in the fine feeder-roots that are important in water and nutrient uptake by the plant. The root galls or knots associated with certain nematode damage to other crops are usually not evident on grasses but may occur in some cases (Figure 12).

Credit: W. T. Crow, UF/IFAS Entomology and Nematology Department
Credit: R. A. Dunn, UF/IFAS Entomology and Nematology Department
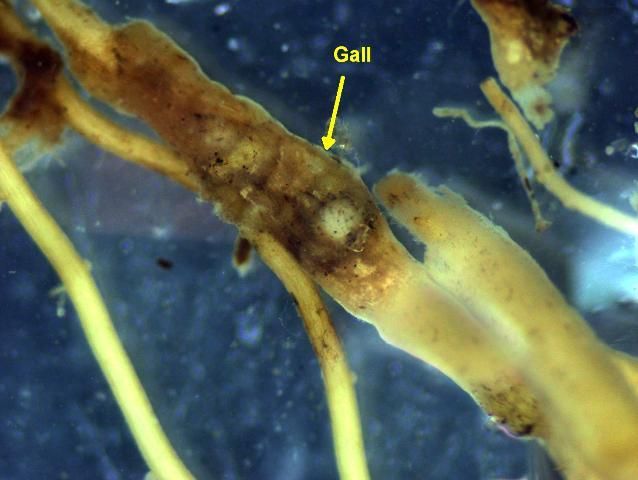
Credit: W. T. Crow, UF/IFAS Entomology and Nematology Department
With any plant problem, having an accurate diagnosis is important to address the problem and to avoid making unnecessary pesticide applications and wasting time, money, and effort. The only reliable way to determine if plant-parasitic nematodes are involved in a grass problem is by having a nematode assay conducted by a professional nematode diagnostic lab. The University of Florida Nematode Assay Lab (NAL) is such a facility. Forms and instructions for submitting nematode samples to the NAL can be found on their webpage http://entnemdept.ufl.edu/nematology-assay-lab/.
A nematode assay is a separate procedure and requires different sampling guidelines than those required for soil analysis or disease samples. Be aware that when a disease sample is submitted to most labs a nematode analysis is not normally performed unless you specifically request it. A nematode assay often requires separate payment and may even be sent to a separate address. Familiarize yourself with the procedures required by the lab where you intend to submit the sample. The accuracy of the diagnosis depends on the quality of the sample that you submit. Instructions for collecting samples for extraction of nematodes from soil are as follows. A video on how to collect nematode soil samples from a golf green can be viewed at https://www.youtube.com/watch?v=frVnM5NpsGY.
- A sample must consist of multiple cores. Nematodes are not evenly distributed in soil, but rather are clumped in distribution. A nematode population density may be high at one spot and low just a few feet way. By collecting multiple cores with a T-type soil probe or similar device, an average population density can be measured. A cup-cutter core is often adequate for a disease diagnosis, but not for nematode diagnosis. A good rule of thumb is to collect 16–20 ½-inch-diameter cores per area (green, fairway, etc.).
- If damage is evident, then sample near the margin of affected areas. Nematode populations will decline in severely damaged areas because they have nothing left to eat. Do not take samples from dead areas. Try to sample turf that is declining, but not dead.
When taking samples from turf that is not yet showing aboveground symptoms, or if sampling before planting, sample in a zig-zag pattern across the area.
3. Put the soil from each sampled area into a plastic bag and seal the bag to preserve the soil moisture. (Sampling for nematodes is different from sampling for nutrient analysis, where dry soil is preferred. Nematodes require moisture to survive, so drying the soil will kill them.)Make sure that each bag is labeled with a permanent marker so that the diagnosis can be assigned to the correct area. If you use a ziplock type bag, seal it with tape because the zippers often come open in transit.
4. Handle samples carefully and do not expose them to direct sunlight or heat. Nematodes are sensitive to high temperatures and UV light. Leaving samples on the dashboard of your car or in the back of a golf cart for periods of time can kill them quickly and make it impossible to accurately diagnose your problem. It is best to keep the nematodes samples in an air-conditioned room until shipping. For shipping and transport, pack the samples well to minimize shifting.
5. Submit the sample right away. Next-day delivery is best. One study found greatest nematode recovery from hand-delivered samples, the next highest from next-day delivery, and the lowest from regular postal delivery. Ship samples early in the week so that they will not sit in a warehouse or on a truck over the weekend.
Root-knot nematodes are very damaging to golf greens in Florida. Soil extraction is not very accurate for diagnosis of these nematodes, so the University of Florida Nematode Assay Lab has a separate procedure for diagnosis of root-knot nematodes from golf greens. A video on how to collect samples for mist extraction of root-knot nematodes from turf plugs can be viewed at https://www.youtube.com/watch?v=Fk1sAcwC760. Using a 1.5-inch-diameter or larger device collect four 2-inch-deep turf plugs from symptomatic areas on the green and place into a plastic bag. Shipping and handling are then as described above.
The staff at the NAL will determine the turf's risk of nematode damage based on which nematodes are found and how many of them there are. Turf from each sample will be assigned a low, moderate, or high risk of damage. Not all plant-parasitic nematodes are equal in their ability to harm grass. For example, one sting nematode can cause damage equal to hundreds of individuals of some other types of plant-parasitic nematodes.
Be aware that different diagnostic labs may use different extraction techniques, use different quantities of soil, or use different thresholds. Because of this, samples submitted to separate labs may report different quantities of nematodes. Do not be alarmed by this; in most cases, the different thresholds used are adjusted to account for the differences in methodology and sample size.
Nematode Management
Before Planting
It is always preferable to avoid a potential problem than to deal with an existing one, so it is best to consider nematodes during course construction or reconstruction. Currently, chemical management of nematodes before planting is most commonly achieved by soil fumigation. Soil fumigation involves injecting a liquid or incorporating a granular material into the soil. The material then either converts to a gas or releases a gas that kills nematodes and other organisms. In addition to nematodes, many of the fumigants have activity against weeds and/or soilborne diseases and/or insects. Several soil fumigants are currently available for course construction and reconstruction including dazomet (Basamid® G), and metam sodium (Vapam®).
Contaminated planting material (sod or sprigs) allows nematodes to spread into new areas. Certified sod is generally not nematode-free. Before purchasing sod, it is sometimes a good idea to have the sod field sampled to detect damaging nematode species.
Research at the University of Florida has found that incorporation of certain organic amendments such as Comand® compost can improve tolerance to nematodes and in some cases suppress nematode numbers.
A key component of Integrated Pest Management (IPM) is the use of resistant or tolerant plants. Research at the University of Florida has found that the dwarf bermudagrass cultivars 'Tifdwarf' and 'Emerald Dwarf' are more tolerant of sting nematodes than the ultradwarf cultivars evaluated. Similarly, the non-dwarf bermudagrass cultivars 'TifTuff' 'TifSport', 'Celebration', and 'Princess 77' were better at withstanding attack by sting nematode than were other cultivars evaluated. Generally speaking, the more vigorously rooting a cultivar, the more nematode-tolerant it should be.
Established Turf
Cultural Practices
Plant-parasitic nematodes are often one of many stress factors affecting the health of turf. The nematodes may be a major contributing factor to turf stress, or a minor one. Often, reducing the overall stress level on turf can help the grass to counteract the negative effects of nematodes.
Mowing height: As a rule of thumb, the lower the grass is mowed, the greater the stress the grass is under. Research has shown that nematode-infested turf declines more readily at lower mowing heights. Often, raising mowing height slightly can reduce nematode damage considerably. Frequent requests for increased ball speed can pressure course superintendents to lower mowing height, but on nematode-infested greens, it's usually wise to sacrifice some ball speed in order to maintain turf.
Fertility: Excessive nitrogen fertilization can increase succulent root growth and encourage rapid foliage growth. Succulent root tips are more susceptible to nematode damage, and the proliferation of root tips (nematode food) can cause nematode population densities to rise dramatically. Rapidly growing foliage drains nutrient reserves from the roots that are needed to compensate for the nematode damage. Under-fertilization should also be avoided. Roots damaged by nematodes will already have a reduced capability to extract nutrients from soil. This makes nutrient deficiencies more pronounced on nematode-infested plants.
Watering: Deep, infrequent watering encourages deep root growth. A deep root system is more tolerant of nematodes than a shallow root system resulting from shallow, frequent watering. However, once nematode damage is extensive, frequent light watering may be required to keep the grass from wilting. During times of extended drought, this can lead to buildup of salts and other problems, so periodic deep irrigation will still be required.
Compaction and aeration: Over-compaction reduces oxygen penetration to the root system and enhances susceptibility to nematode damage. Aeration encourages a healthy root system and thereby enhances tolerance to nematodes. In cases where certain greens are nematode-infested and others are not, aerate the infested greens last to avoid transferring nematodes to the clean ones in soil adhering to aerification equipment.
Soil amendments: Generally, anything that promotes healthy root growth can enhance tolerance to nematodes. Incorporation of colloidal phosphate has been shown to enhance bermudagrass tolerance to several nematodes. Top-dressing with certain organic amendments such as Comand® compost may also reduce nematode damage and speed the recovery process after damage has occurred.
Shade: By damaging roots, nematodes impair the ability of turf to store energy. Therefore, nematode-damaged turf often is more prone to decline from shade, or prolonged poor weather. If greens are in partial shade, trimming or thinning trees to get more light to the turf will greatly enhance the turf's ability to withstand nematode damage.
Overseeding: UF research has shown that overseeding can increase nematode numbers on bermudagrass during transition by providing an alternate food source to the nematodes during the winter. Therefore, from a nematode management point of view it is best to avoid overseeding.
Bionematicides
There are several bionematides available for turf use. Many of these bionematicides are plant-derived, others come from animals, and still others are either live microorganisms or are compounds produced by microorganisms. For most of these we do not have enough data to confidently say they are effective or not.
Zelto®+Crescendo®: Research conducted at the University of Florida has shown a combination of two bionematicides, Zelto and Crescendo, is effective against root-knot nematode on turf. These are not live microorganisms, but their active ingredients are produced by live bacteria grown commercially in vats. Best results were achieved by tank-mixing the two products and applying monthly for several months.
Chemical Nematicides
Even the best-managed turf can suffer from nematode injury, requiring chemical intervention. Historically, most nematicides have been toxic at low levels and water soluble in order to move into the soil profile and kill nematodes. Many of the effective nematicides used in the past were withdrawn from the market for environmental and health reasons. The new nematicides are much safer and carry less risk for negative environmental impact than the older classes of nematodes, but still should be used with care. When using any nematicide, follow the label instructions strictly to minimize human and environmental health impacts and to avoid liability. Nematicides labeled for golf course turf in Florida that have shown consistent efficacy in University of Florida research trials are discussed below. This information is not a substitute for the product label. Always follow directions on the product label when applying any pesticide. There is no single nematicide that is best to use in all situations and against all kinds of nematodes. Therefore, monitoring nematode populations through sampling and selecting products based on their expected efficacy on the nematodes present is important.
Abamectin products: Abamectin is the active ingredient in several turfgrass nematicides including: Divanem SC (Syngenta), TODAL (Quali-Pro), and Nemamectin (RightLine). Abamectin was a pesticide originally isolated from a soil bacterium. Abamectin binds to thatch and organic matter, so it works best against nematodes that inhabit the thatch and upper soil layer, particularly root-knot nematodes, although it can help manage other types of nematodes as well. For all of these abamectin products, a maximum of 0.27 lb. of abamectin per acre can be applied per year, with a maximum of 0.0675 lb. abamectin per acre per application. However, small areas (10,000 ft2 or less) may be treated up to 4 times with 0.27 lb. The concentration of abamectin varies among products, so read the product label to determine the formulation rates to apply. Abamectin nematicides are restricted-use pesticides for golf and professional and collegiate athletic turf only. In University of Florida trials abamectin has performed well when either applied four times at 4-week intervals at the maximum single application rate or eight times at half the maximum single-application rate at 2-week intervals. For best results, it is recommended that abamectin products be tank-mixed with a soil penetrant for application, and that it be irrigated with up to ¼-inch of water immediately after application.
Fluopyram: Fluopyram is a succinate dehydrogenase inhibitor (SDHI) nematicide and fungicide. University of Florida research results reveal that fluopyram is extremely effective against sting and root-knot nematodes on turf, but is ineffective against lance nematode. There are currently two fluopyram pesticides labeled for nematode management, Indemnify® and Resilia®. Fluopyram is the only active ingredient in Indemnify, while Resilia contains fluopyram along with two other fungicides. University of Florida research indicates that while Resilia contains less fluopyram than Indemnify, it provides good control of root-knot nematodes on turf. However, for sting nematode management the higher dosage of fluopyram in Indemnify will yield better results.
Fluensulphone: The active ingredient in Nimitz Pro G® is fluensulfone, a nematicide in its own chemical class. This formulation is a granular product applied to the turf surface using a spreader and then moved into the soil with irrigation. Fluensulfone has both contact and systemic activity, so it is effective against nematodes in soil and inside roots, including lance nematode. Nimitz Pro G is labeled for all warm season turf uses, including golf course, athletic field, and lawn. It is not labeled for use on cool-season grasses due to phytotoxicity. A maximum of 240 pounds per acre per year may be applied, but this can be broken up into multiple applications of lower doses. In University of Florida trials best results have been achieved with either 4 monthly applications of 60 pounds per acre or 3 monthly applications of 80 pounds per acre.
Curfew Soil Fumigant®: Curfew Soil Fumigant® is different from most other turfgrass pesticides in that it is injected into the soil profile as a liquid that then volatilizes and moves through the soil as a gas (fumigant). The active ingredient in Curfew Soil Fumigant is 1,3-dichloropropene (1,3-D). Curfew Soil Fumigant cannot be applied by golf course staff, but may only be applied by approved custom applicators. Applications are scheduled through certain golf course industry distributors. While application equipment for greens is slightly different from that used on fairways, the application method is similar. Slits are made in the turf by knives that have a metal drip tube welded onto the back. As the knives are pulled through the soil, the fumigant is injected 5–6 inches deep into the soil profile, and the slits are then pressed back together to reduce fumigant loss. The 1,3-D turns into a gas that disperses through the soil profile and kills nematodes on contact.
Curfew Soil Fumigant is highly effective against sting nematode and other ectoparasites. It also is effective against mole crickets. However, because it is a contact nematicide, its efficacy against endoparasitic nematodes such as lance or root-knot nematodes is less consistent. Dow AgroSciences instructions for irrigation and turf care following a Curfew Soil Fumigant application should be followed closely to avoid problems and maximize the benefits of Curfew. Curfew cannot be applied within 30 ft of buildings, and a 24-hour reentry restriction applies. In areas of Florida with certain geologic features, Curfew Soil Fumigant cannot be used.
Other Products: In order for University of Florida faculty to recommend a pest management product, data from properly conducted field research trials should indicate that the product works on a consistent basis. There are a several botanical nematicides and microbial products on the market. For most of these, either field efficacy data is lacking, or field trial data has not shown consistent benefit. That does not mean that these products never work, but that there is insufficient evidence to recommend their use.
Nematicide Resistance: Research at University of Florida has identified resistance to fluopyram in root-knot and sting nematode populations from golf courses that having been using this nematicide regularly for multiple years. Therefore, it is very important to rotate nematicide modes of action to maintain the efficacy of this powerful nematode management tool. Refer to Table 1 below for assistance in selecting effective nematicide rotation options.
Table 1. The relative performance in University of Florida research of the different nematicides and bionematicides described herein on the three most important types of nematodes affecting golf turf in Florida. As much as possible rotate nematicides from different groups to avoid nematicide resistance.
Summary
Nematode management on a golf course can be a daunting task. Expectations for a pristine playing surface are high, and nematodes are notoriously difficult to control. The best management practices for golf courses with nematode problems are 1) avoid other stresses on the grass as much as possible, 2) monitor nematode populations by sampling frequently, and 3) apply nematicides when needed.
The University of Florida is committed to bringing you the most current information possible. Consequently this document will be modified with each breaking development. The most current version of this document may be obtained online at the UF/IFAS Extension Electronic Document Information System (EDIS) website at https://edis.ifas.ufl.edu/.
For additional information regarding nematodes, nematode management, or help interpreting nematode assay results contact:
William T. (Billy) Crow, Ph.D.
Professor of Nematology
UF/IFAS Entomology and Nematology Department
PO Box 110620
Gainesville, FL 32611
(352) 273-3941
FAX: (352) 392-0190
E-mail: wtcr@ufl.edu
For information on submitting samples to the University of Florida Nematode Assay Lab or to check on the status of a sample you submitted, contact:
UF/IFAS Nematode Assay Lab
1881 Natural Area Drive
PO Box 110620
Gainesville, FL 32611
(352) 392-1994
FAX: (352) 392-0190
E-mail: nemalab@ifas.ufl.edu