Introduction
The salvinia weevil, Cyrtobagous salviniae (Calder & Sands) (Figure 1), is a subaquatic (underwater) herbivorous insect native to Brazil (Calder and Sands 1985). This insect feeds on the invasive aquatic plants Salvinia molesta D.S. Mitchell and Salvinia minima (Baker). This insect is an effective classical biological control agent used in several countries to control the invasive giant salvinia, Salvinia molesta. Feeding by Cyrtobagous salviniae larvae and adults kills its invasive host plants and restores recreational, agricultural, and ecosystem functions in aquatic systems. In the United states, the insect has been credited for controlling Salvinia minima in Florida (Jacono et al. 2001) and causing the decline of Salvinia molesta in Texas and Louisiana (Tipping et al. 2008).

Credit: Katherine Parys, USDA-ARS, Bugwood.org
Distribution
Cyrtobagous salviniae is native to southern Brazil. However, it now occurs in at least 17 other countries, including Australia, Papua New Guinea, Sri Lanka, South Africa, and the United States (Winston et al. 2014). The insect was deliberately introduced into these countries as a classical biological control agent of the invasive aquatic weed, Salvinia molesta (Room et al. 1981; Cilliers 1991; Tipping et al. 2008; Russell et al. 2017). In the United States, Cyrtobagous salviniae was introduced into Texas and Louisiana from Brazil in a project that began in 1999 for management of Salvinia molesta. Florida has an established adventive population of Cyrtobagous salviniae, which was probably introduced in the 1960s or earlier (Kissinger 1966). It is now present in more than 77% of the Florida's waterbodies, resulting in the control of Salvinia minima (Jacono et al. 2001).
Description
Adult and immature life stages of this beetle can be found on Salvinia molesta or Salvinia minima above and below the water surface. Interestingly, these insects can breathe underwater through an air bubble (called a plastron) that they create and attach to the underside of their body (Forno et al. 1983; Russell et al. 2016).
Eggs
The eggs of Cyrtobagous salviniae are deposited singly on the leaves or rhizomes (belowground or sub-surface stems) of Salvinia molesta or Salvinia minima. Eggs are elliptical, milky white, and measure 0.5 by 0.2 mm (Figure 2).

Credit: Alana Russell, LSU AgCenter
Larvae
The larvae of Cyrtobagous salviniae undergo five instars, or developmental stages. Larvae progress in size from a 1 mm long first instar to a 2.6 mm long fifth instar. Their color is milky white with a distinct hardened, dark brown head capsule (Figure 3). Early instars feed on new leaf buds of Salvinia molesta or Salvinia minima, while later instars bore into the rhizome and feed internally on new rhizomes. Mature larvae develop into a resting pupal stage, during which they transform into adult beetles.

Credit: Alana Russell, LSU AgCenter
Pupae
The pupae of Cyrtobagous salviniae measure 2 mm by 2.6 mm and remain within an enclosed cocoon made with Salvinia molesta or Salvinia minima roots. They pupate underwater within rhizomes of Salvinia molesta or Salvinia minima.
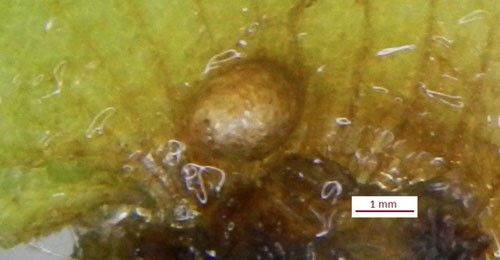
Credit: Alana Russell, LSU AgCenter
Adult
The adults of Cyrtobagous salviniae measure 2 to 3.5 mm long and are brown during the first five days following emergence before turning shiny black for the remainder of their life. Similar to other weevil species, they have hardened forewings (called elytra) and membranous hindwings used for flight, along with a long snout on their head (Figure 5). The antennae and chewing mouthparts are located at the apex of the rostrum (snout) as shown in Figure 5.

Credit: Alana Russell, LSU AgCenter
Life Cycle and Biology
Cyrtobagous salviniae is a multivoltine species; it has multiple generations per year. The insect undergoes complete metamorphosis with four distinct developmental stages: eggs, larvae, pupae, and adults. The developmental time from egg to adult takes approximately 45 days but may vary depending on temperature and food quality. Adult females oviposit (deposit eggs) in small fissures or crevices that they cut into the new growth of Salvinia molesta or Salvinia minima. After approximately 10 days, a larva will emerge from the egg and feed for approximately 23 days before developing into a pupa. Pupal development takes 10 to 15 days, after which an adult beetle will eclose (emerge) to begin feeding and reproducing. Newly emerged females begin laying eggs approximately five days after emerging from the pupa. A single female can deposit one egg every two to five days for at least 60 days (Eisenberg et al. 2018).
Both larval and adult stages feed on plant material. Neonates (newly emerged larvae) feed on the roots within their first to fourth days and move to new leaf buds within five to nine days after hatching (Sands et al. 1983). After feeding on external plant buds and roots, the larva will tunnel into the rhizomes and feed internally (Sands et al. 1983, Julien et al. 1987). Tunneling into the rhizomes is essential for the larval stage to complete development (Sands et al. 1983, Julien et al. 1987). Adults feed on roots, buds, and young leaves that enclose the buds (Julien et al. 1987). Nitrogen-rich host plants promote larval development and increased egg production (Sands et al. 1986).
Host
Field studies in Brazil found that Cyrtobagous salviniae only feeds on plants from the genus Salvinia, commonly called floating ferns. These plants consist of rhizomes that run horizontally below the water surface and produce leaves in whorls or spiral patterns of three. In the United States, Cyrtobagous salviniae feeds exclusively on two invasive floating fern species, Salvinia molesta and Salvinia minima. These two species are the only members of the genus Salvinia in North America (Jacono et al. 2001, Russell et al. 2016). Salvinia molesta, native to southeastern Brazil, is the primary host of Cyrtobagous salviniae and a major target of weed biological control programs around the world.
In the United States, Salvinia molesta was first observed in South Carolina in 1995, followed by Louisiana in 1998, and several other states by 1999 (Center for Agriculture and Bioscience International 2018). The plant is now well established in Louisiana and Texas (Tipping et al. 2008). As of 2019, Salvinia molesta is on the Federal Noxious Weed List, in an effort to increase awareness and reduce the spread of noxious weeds. Additionally, the second species, Salvinia minima, was introduced to North America in late 1920s and has been reported to occur in Florida, Texas, and Louisiana. Because of the early introduction and pressure caused by Cyrtobagous salviniae, Salvinia minima is not considered a problem in Florida, and the impacts in Texas and Louisiana are not well documented (Jacono et al. 2001).
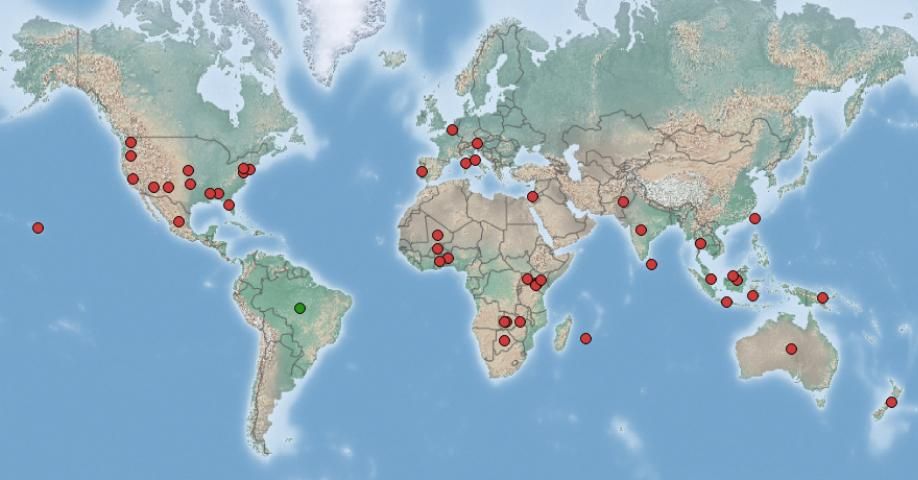
Credit: Center for Agriculture and Bioscience International, 2018. (Available online at: https://www.cabi.org/isc/datasheet/48447)
Economic Importance
Salvinia molesta is considered the most invasive Salvinia species and a serious threat to tropical and subtropical aquatic ecosystems worldwide (Douglas-Oliver 1993). Salvinia molesta spreads from fragments of leaves and roots via water currents and on contaminated equipment such as boats and vehicles. It has been found forming dense mats on water surfaces that can thicken up to 1 m deep (Thomas and Room 1986). Beginning in the 1940s, human activities have spread Salvinia molesta to various tropical and subtropical regions of Africa, Asia, Australia, and United States (Figure 6, Thomas and Room 1986). The plant now occurs in more than 30 countries.
Cyrtobagous salviniae is an important classical biological control agent of Salvinia molesta worldwide. Salvinia molesta is considered a major destructive aquatic weed in waterbodies characterized by slow-moving fresh water. Dense stands of Salvinia molesta not only disrupt recreational activities like boating and fishing, but also displace native flora, clog irrigation systems, and deplete dissolved oxygen in water bodies, causing hypoxic conditions that kill aquatic wildlife (Horner 2002; Russell et al. 2017). Feeding damage by Cyrtobagous salviniae suppresses the growth of Salvinia molesta. Larval feeding in particular is the most damaging because it destroys the vascular tissue and interrupts the translocation of nutrients, causing the plant to decay, lose buoyancy, and sink to the bottom of water bodies (Julien et al. 2002; Russell et al. 2016).
Because of the insect's effect on Salvinia molesta, it has been introduced as a biological control agent in multiple countries around the world with the intent of controlling the invasive plant. For example, Cyrtobagous salviniae successfully eliminated a Salvinia molesta infestation within 15 months after being released into Lake Moondarra, Australia in 1980. Introduction of Cyrtobagous salviniae to at least 17 other countries has achieved similar levels of control within 36 months (Julien et al. 2009; Julien et al. 2012). In the US, evaluation of the impact of this insect in Texas and Louisiana revealed that it has the capacity to reduce the surface coverage of Salvinia molesta by more than 99% (Tipping et al. 2008).
Selected References
Calder AA, Sands DPA. 1985. "A new Brazilian Cyrtobagous hustache (Coleoptera: Curculionidae) introduced into Australia to control salvinia." Austral Entomology 24: 57–64.
Center for Agriculture and Bioscience International. 2018. Salvinia molesta (Kariba weed). In: Invasive species compendium. Retrieved from: https://www.cabi.org/isc/datasheet/48447 United States Department of Agriculture, Stoneville, MS. (20 March 2019)
Cilliers CJ. 1991. "Biological control of water fern, Salvinia molesta (Salviniaceae), in South Africa." Agriculture, Ecosystems and Environment 37: 219–224.
Douglas-Oliver J. 1993. "A review of the biology of giant salvinia (Salvinia molesta Mitchell)." Journal of Aquatic Plant Management 31: 227–231.
Eisenberg L, Johnson S, Grodowitz MJ. 2018. "The reproductive morphology and physiological age grading of the female salvinia weevil, Cyrtobagous salviniae Calder and Sands." International Journal of Insect Science 10: 1–8.
Forno IW, Sand DPA, Sexton W. 1983. "Distribution, biology, and host specificity of Cyrtobagous singularis Hustache (Coleoptera: Curculionidae) for the biological control of Salvinia molesta." Bulletin of Entomological Research 73: 85–95.
Horner T. 2002. Field release of the salvinia weevil, Cyrtobagous salviniae Calder and Sands (Curculionidae: Coleoptera) for control of giant salvinia, Salvinia molesta Mitchell (Hydropteridales: Salviniaceae). Retrieved from: http://www.salvinia.org/LCR/salviniaEA2.pdf United States Department of Agriculture, Riverdale, MD. (20 March 2019)
Jacono CC, Davern TR, Center TD. 2001. "The adventive status of Salvinia minima and S. molesta in the southern United States and the related distribution of the weevil Cyrtobagous salviniae." Castanea 66: 214–226.
Julien MH, Bourne AS, Chan RR. 1987. "Effects of adult and larval Cyrtobagous salviniae on the floating weed Salvinia molesta." Journal of Applied Ecology 24: 935–944.
Julien MH, Center TD, Tipping PW. 2002. Floating fern (Salvinia), pp. 17–32. In Van Driesche RG, Blossey B, Hoddle M, Lyon S, Reardon R (editors). Biological Control of Invasive Plants in the Eastern United States. FHTET-2002-04, USDA Forest Service, Morgantown, WV.
Julien MH, Hill MP, Tipping PW. 2009. Salvinia molesta D. S. Mitchell (Salviniaceae), pp. 378–407. In Muniappan R, Reddy GV, Raman A (editors.). Biological Control of Tropical Weeds Using Arthropods. Cambridge, UK: Cambridge University Press.
Julien MH, McFadyen RE, Cullen JM. 2012. Biological Control of Weeds in Australia. Melbourne, Australia: CSIRO. 648p.
Kissinger DG. 1966. "Cyrtobagous Hustache, a genus of weevils new to the United States fauna (Coleoptera: Curculionidae: Bagoini)." The Coleopterists' Bulletin 20: 125–127.
Room PM, Harley KLS, Forno IW, Sands DPA. 1981. "Successful biological control of the floating weed salvinia." Nature 294: 78–80.
Russell A, Johnson S, Mudge C, Diaz R. 2016. The biology and ecology of the salvinia weevil: A biological control agent for the management of giant salvinia. Retrieved from: https://www.lsu.edu/departments/entomology/assets/salviniaweevil.pdf Pub. 3474 Louisiana State University, Baton Rouge, LA. (14 February 2022)
Russell A, Johnson S. Cibilis X, McKay F, Moshman L, Madeira P, Blair Z, Diaz R. 2017. "Surveys in Argentina and Uruguay reveal Cyrtobagous salviniae (Coleoptera: Curculionidae) populations adapted to survive temperate climates in southeastern USA." Biological Control 107: 41–49.
Sands DPA, Schotz M, Bourne AS. 1983. "The feeding characteristics and development of larvae of a salvinia weevil Cyrtobagous sp." Entomologia Experimentalis et Applicata 34: 291–296.
Sands DPA, Schotz M, Bourne AS. 1986. "A comparative study on the intrinsic rates of increase of Cyrtobagous singularis and C. salviniae (Coleoptera: Curculionidae) on the water weed Salvinia molesta." Entomologia Experimentalis et Applicata 42: 231–237.
Thomas PA, Room PM. 1986. "Taxonomy and control of Salvinia molesta." Nature 320: 581–584.
Tipping PW, Martin MR, Center TD, Davern TM. 2008. "Suppression of Salvinia molesta Mitchell in Texas and Louisiana by Cyrtobagous salviniae Calder and Sands." Aquatic Botany 88: 196–202.
Winston RL, Schwarzländer M, Hinz HL, Day MD, Cock MJW, Julien MH. (Eds.) 2014. Biological Control of Weeds: A World Catalogue of Agents and Their Target Weeds, 5th edition. USDA Forest Service, Forest Health Technology Enterprise Team. Morgantown, WV. 838 pp.