The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Psorophora columbiae is a member of the broader Psorophora confinnis species complex (a group of closely related species) that occurs across much of North and South America. This mosquito is associated with sun-exposed ephemeral water sources such as pooled water in agricultural lands and disturbed or grassy landscapes. The ubiquity of these habitats among agrarian and peridomestic landscapes contribute to explosive abundance of Psorophora columbiae following periods of high precipitation. Psorophora columbiae is both a common nuisance mosquito and significant livestock pest.
Common names of Psorophora columbiae vary by region. In rice-growing regions of Arkansas, Florida, and Louisiana Psorophora columbiae is known as the dark rice field mosquito because of its overall dark coloration and proliferation in flooded rice fields. In the Atlantic Seaboard region Psorophora columbiae is colloquially referred to as the glades mosquito or the Florida glades mosquito (King et al. 1960) due to its association with grasslands (glades) in otherwise forested areas.
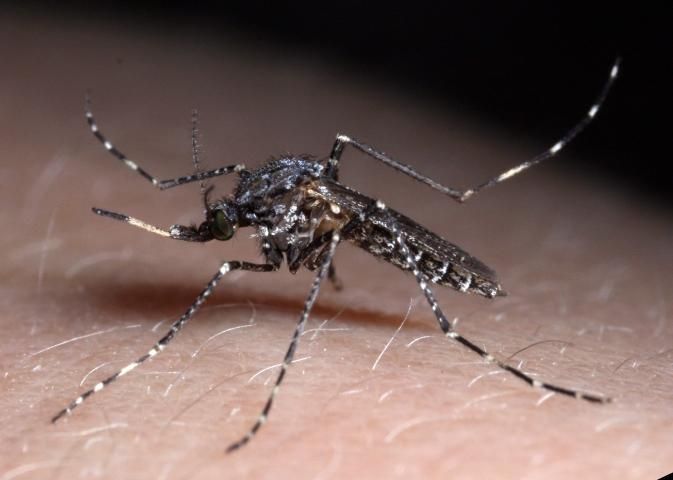
Credit: Nathan Burkett-Cadena, UF/IFAS
Synonymy
Janthinosoma columbiae Dyar & Knab (1906)
Janthinosoma floridense Dyar & Knab (1906)
Janthinosoma texanum Dyar & Knab (1906)
From the Integrated Taxonomic Information System and International Commission on Zoological Nomenclature.
Distribution
Psorophora columbiae belongs to a complex of similar species containing multiple forms of the namesake species (Moncayo et al. 2008), and it is also a representative of an interbreeding group of closely related species that includes Psorophora confinnis throughout South America, Psorophora funiculus in the northern border region of Colombia and Panama, Psorophora toltecum along the Pacific coastal regions of the southwestern US and Mexico, and Psorophora columbiae, concentrated around the Gulf Coast and Atlantic Seaboard states of the US (Lanzaro et al. 2015). Functionally, there are no morphological and few other differences between members of the Psorophora columbiae complex and the parent Psorophora confinnis complex (Moncayo et al. 2008; Lanzaro et al. 2015). As a result, the distributions of complex members, including Psorophora columbiae, are typically used in taxonomic identification rather than morphology (Figure2). Taxonomy within the Psorophora confinnis complex is currently in a state of flux; however, contemporary population genetics investigations support the recognition of four distinct species (Lanzaro et al. 2015).
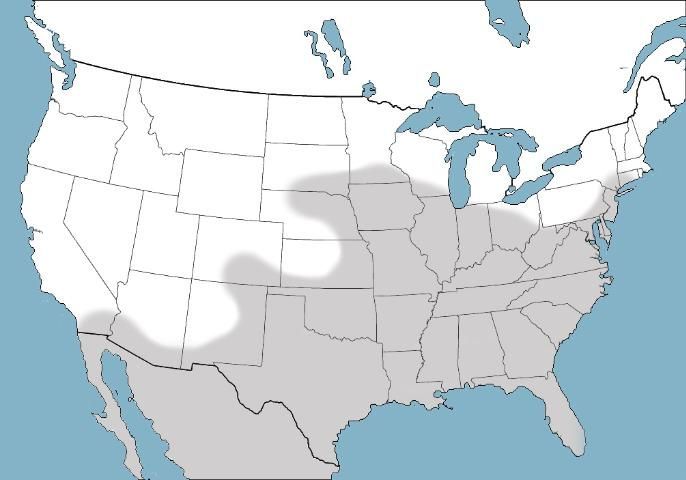
Credit: Nathan Burkett-Cadena, UF/IFAS
Psorophora columbiae often reach peak abundance in June and July, depending on the availability of suitable larval habitats (Bolling et al. 2005). Rain within a preceding two-week period and a lack of canopy cover over standing water are strongly associated with rapid population growth of these species (Bolling et al. 2005), especially for the two closest in form, Psorophora columbiae and Psorophora toltecum, throughout the southern and eastern US (Ruiz-Garcia et al. 2006).
Description
Adults
Adults of Psorophora columbiae are generally medium-sized mosquitoes (Burkett-Cadena 2013), with wing length approximately 4.0–4.5 mm (Carpenter and LaCasse 1955). The body tends to be dark brown, dark gray, or dull black in color with numerous patches of pale scales (Figure 1). Diagnostic characters include a broad band of pale scales on the proboscis (Figure 3); palps with white scales at the apex (Figure 3); patches of pale golden scales on the apical half of abdominal segments (Figure 4); dark and pale wing scales in no definite pattern (Figure 5); and pale bands at the base of each tarsal segment, in the middle of the first tarsal segment, and near the apex of the femur (Figure 6). Males are differentiated from females by having plumose, or noticeably feather-like, antennae as opposed to the wire-like antennae of the female.
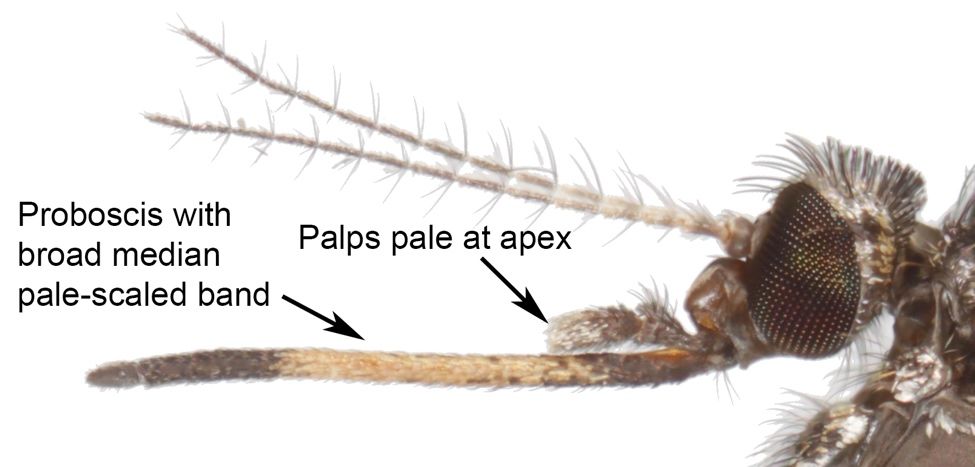
Credit: Nathan Burkett-Cadena

Credit: Nathan Burkett-Cadena

Credit: Nathan Burkett-Cadena
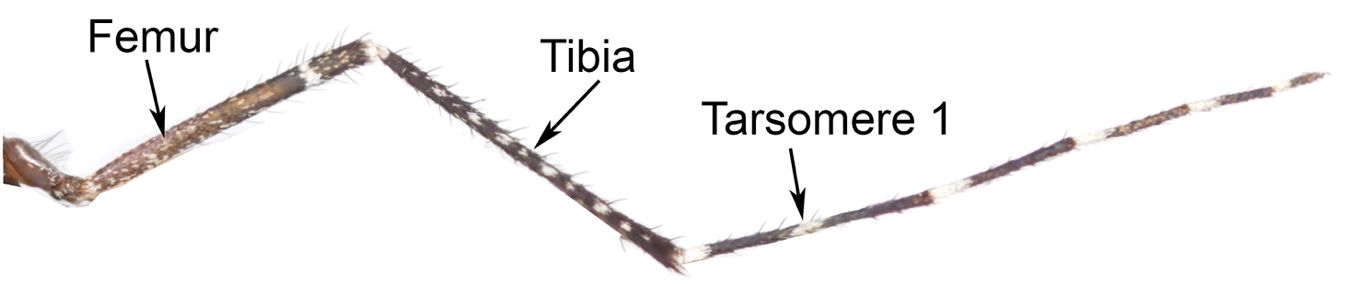
Credit: UF/IFAS
Pupae
As with other mosquitoes, pupae of Psorophora columbiae rest at the surface of the water with the aid of an air pocket within the underside of the cuticle (Foster and Walker 2018). Gas exchange occurs through respiratory trumpets that arise from the dorsolateral surface of the thorax, above the eyes (Figure 7). The pupa is mobile, quickly escaping down into the water column when disturbed. The pupal stage of Psorophora columbiae is relatively short, with only 36–48 hours in this stage before emerging as an adult (Foster and Walker 2018). Generally, Psorophora pupae can have one or more of the following characters that help distinguish them from Aedes, Culex, and Culiseta: spines on the posterior corner of the fourth abdominal segment, lobes on the posterior edge of the eighth abdominal segment, and/or a seta (hair-like structure) arising from the paddle on the posterior tip of the pupa (Figure 8, Barr and Barr 1969). The Psorophora confinnis complex tends to bear a diagnostic feature that the seta arising from the paddle is no more than 1/6 the length of the paddle it is attached to, appearing relatively short in length for that particular seta when compared to other members of the genus (Figure 8, Barr and Barr 1969).
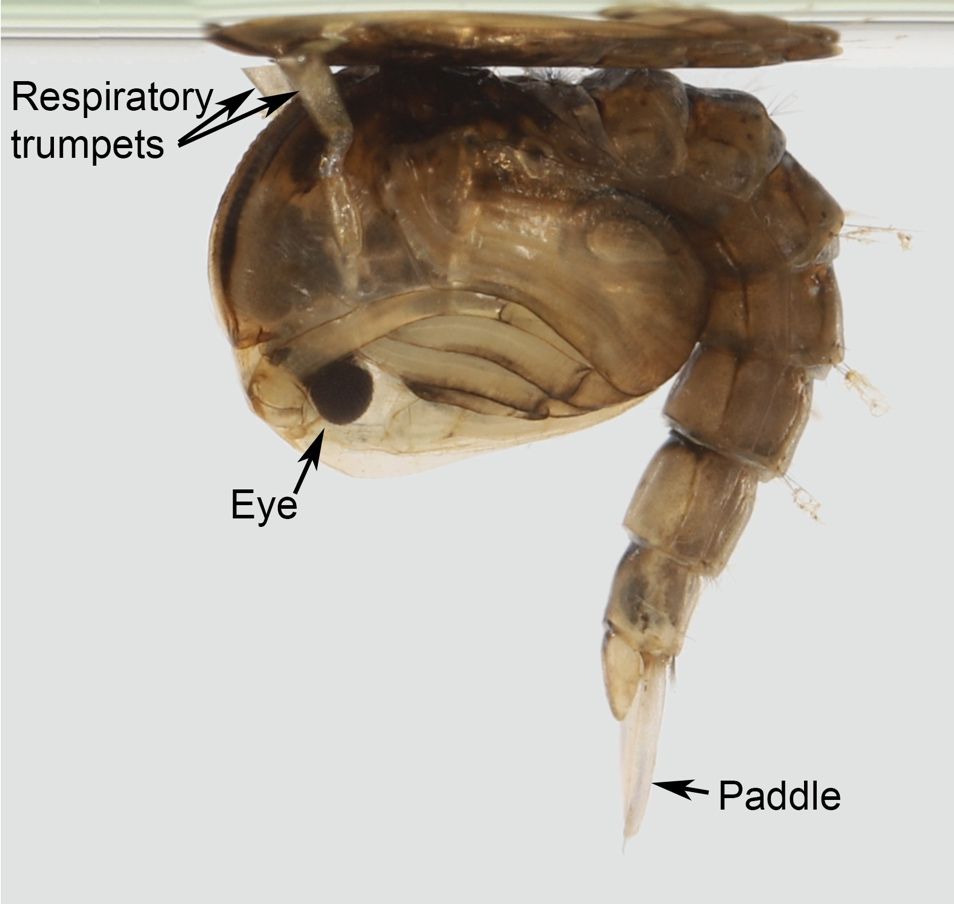
Credit: Nathan Burkett-Cadena
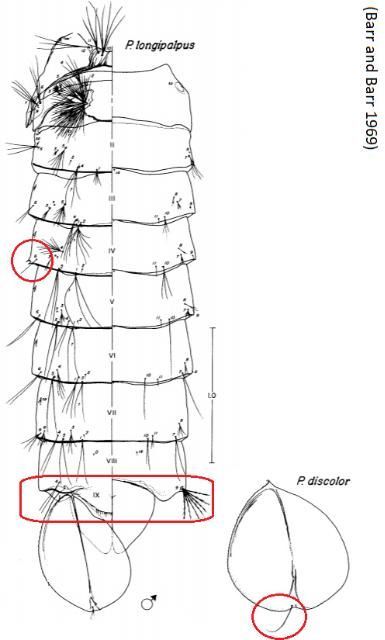
Credit: Barr and Barr (1969)
Larvae
Larvae of Psorophora columbiae are typically large in size when fully grown but vary in color from pale to dark in shades of gray or brown (Figure 9). The head is somewhat rectangular in shape, and wider than long (Figure 9). The antennae are shorter than the length of the head (Figure 10), a feature that is characteristic of most species in the subgenus Grabhamia to which Psorophora columbiae belongs. A cluster of four setae located on the anterior-middle of the head each have more than four branches (Figure 10). The siphon and terminal abdominal segment have important features for identifying mosquito larvae. The comb scales, a series of spiny barbs located on the eighth abdominal segment (Figure 9), are thorn-like, with shorter barbs adjacent to the main spine on each individual scale (Figure 9). Psorophora columbiae has six comb scales on the eight abdominal segment (Figure 9).

Credit: Nathan Burkett-Cadena, UF/IFAS

Credit: Nathan Burkett-Cadena
The anal papillae, or anal gills, arise from the apex of the tenth abdominal segment, surround the anal opening, and are used in osmoregulation and respiration. The anal papillae (two dorsal and two ventral) of Psorophora columbiae (Figure 9) are long, translucent, taper to a point and are longer than the total length of the tenth abdominal segment (Figure 9). The papillae are fragile, frequently breaking off during handling or when preserving larvae in alcohol. If they are not present when examining the larvae for identifying characters, the siphon is the best alternative.
Extending dorsally from the eighth abdominal segment is a sclerotized (hardened) breathing tube called the siphon (Figure 9). The siphon is rigid and has several useful structures for identifying mosquito larvae. Near the base of the slightly bulbous siphon, where it connects to the abdomen, a series of pecten spines (also called teeth) arise from the cuticle (Figure 9). The variation in the number of pecten spines was the first character that implied geographically distinct forms or species of Psorophora columbiae existed (Bickley 1984). For general identification of Psorophora columbiae and the larger Psorophora columbiae species complex, a series of three to six pecten spines will arise from the siphon in succession with relatively equal spacing between them (Figure 9).
Eggs
Eggs of Psorophora columbiae are difficult to find in the natural environment, because they are deposited in humid soil by gravid females. However, they have been described as dark, approximately 0.5 mm x 0.2 mm oblong structures with numerous spine-like tubercles that help adhesion to substrates (Figure 11; Bosworth et al. 1998).
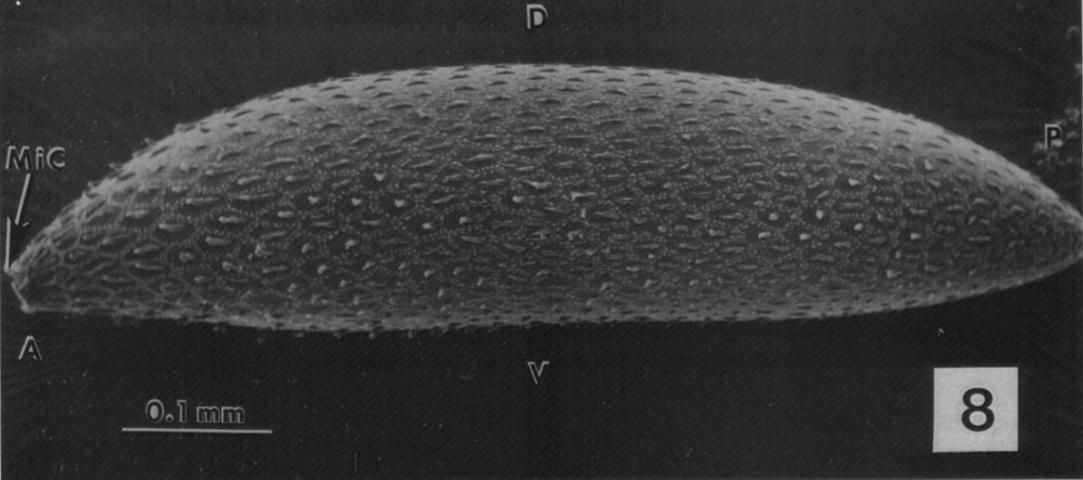
Credit: Bosworth et al. (1998)
Life Cycle and Biology
Psorophora columbiae females deposit eggs primarily on the damp soil of open, sunny locations subject to temporary flooding (Bolling et al. 2005), which can lead to quick colonization of temporary pools of water in open, exposed fields or marshes after rainfall events (Wagner et al. 2007). For example, hatched larvae have been collected from puddles, flooded tire ruts, and grassy swales (Lanzaro et al. 2015).
Regardless, Psorophora columbiae have an ephemeral, floodwater-type biology in the U.S., particularly given that floodplains, runoff areas, and low drainage areas do not retain water permanently (Cleckner et al. 2011). Floodwater mosquitoes quickly occupy temporary standing water, often after the onset of heavy rains and the resulting flooding of low areas that are not necessarily connected to adjoining streams or ponds. As a consequence, the species is frequently found in close association with agricultural wetlands (Botello et al. 2013) such as rice fields or areas of irrigation runoff (Allen et al. 2010). Flooding of arid habitats in southwest US desert and semi-arid transitional lands, particularly from irrigation, can result in population explosions of Psorophora columbiae (Godsey et al. 2012, Britch et al. 2011, Pitzer et al. 2009).
Although not principally a peridomestic mosquito, Psorophora columbiae does occur readily amidst developed areas provided that a larval habitat is located in the area. For example, artificial zoological enclosures intended to replicate wetland habitat, such as for alligators, have been found to actively harbor this mosquito (Unlu et al. 2010). The mosquito also has been found incidentally in water-filled tires, but containers do not represent a significant habitat for the species (Qualls and Mullen 2006).
Courtship and mating occur in flight after adult males have congregated around landmarks in the environment (Peloquin and Olson 1985). Flying males attract females by forming curtain-like or funnel-like swarms that then transition into figure-eight patterns (Peloquin and Olson 1985). As with other mosquitoes, males do not blood feed, instead searching out nectar sources from plants and honeydew (Foster and Walker 2018). Adult females frequently take sugar meals as well (Foster and Walker 2018), but are pestiferous when seeking blood meals to nourish eggs.
Psorophora columbiae has historically been described as preferring to take blood meals from large mammalian hosts, particularly livestock such as cattle (Kuntz et al. 1982). However, the mosquito is known to feed on any mammalian hosts, including humans, within the vicinity of its habitat (Britch et al. 2011, Qualls et al. 2011). Exceptionally large outbreaks may even be linked to zoonotic pathogen transmission (Godsey et al. 2012; Pitzer et al. 2014). While livestock are well-recognized hosts of Psorophora columbiae, blood meal analysis in Florida showed that more Psorophora columbiae blood meals were taken from rabbits (40%) than any other host, including cows (37%) (Edman 1971).
Medical-Veterinary Importance
Psorophora columbiae can carry infective stages of canine heartworm, Dirofilaria immitis (Leidy) (McKay et al. 2013). The mosquito has been implicated in transmission of this parasite in areas where multiple mosquito vector species are active concomitantly (McKay et al. 2013, Paras et al. 2014). Because Psorophora columbiae populations can reach such high levels after large rainfall events, the abundance of Psorophora columbiae has led to its incrimination as a significant heartworm vector species (Paras et al. 2014). Therefore, precautions, such as prophylactic treatments, should be taken for canine heartworm, particularly in the southeastern US. Despite the importance of Psorophora columbiae, as many as 25 species across four different mosquito genera all contribute to the transmission of canine heartworm throughout the same range (Ledesma and Harrington 2012), which reinforces that vectors transmit this pathogen in concert and are not easily targeted for breaking the transmission cycle.
In field investigations, Psorophora columbiae has frequently tested positive for West Nile virus (Unlu et al. 2010), but it has never been implicated as a major epidemic vector responsible for outbreaks of West Nile virus in humans (Godsey et al. 2012). In contrast, Psorophora columbiae is considered to be a fully competent vector (has the ability to transmit) of epidemic strains of Venezuelan equine encephalitis virus (Moncayo et al. 2008). However, the eastern forms of Psorophora columbiae are less susceptible to infection than Psorophora toltecum occurring in Mexico or California (Moncayo et al. 2008).
Rift Valley fever virus is not present in North America, but laboratory infection studies suggest that Psorophora columbiae could likely transmit Rift Valley Fever virus due to abundance of the species and preference for biting large mammals (Turrell et al. 2015). Infections would likely arise from frequency of bites rather than high competence for the virus because Psorophora columbiae possess a salivary gland barrier that limits the amount of virus particles transmitted during a bite (Turell et al. 2015). Large numbers of Psorophora have historically contributed to suffocation, anemia, and occasional transmission of anaplasmosis in cattle (Bishop 1933, Howell et al. 1941).
Management
Habitat identification is a critical first step for management. When ephemeral pools, drainage areas, and shallow floodplains are treated with biorational larvicides such as Bacillus thuringiensis israelensis (Bti) or Spinosad, the emergence of pestiferous, possibly harmful, adults can be virtually eliminated from the targeted area (Allen et al. 2010; 2008). Otherwise, point-source adulticides applied to harborage sites and along structural barriers that intercept the flight path of Psorophora columbiae can effectively reduce human contact with adult mosquitoes (Britch et al. 2011). Treating cattle with topical ectoparasite preventatives, such as a permethrin, can provide a reduction in Psorophora columbiae when the mosquitoes attempt to feed on the livestock (Nasci et al. 1990).
In the aftermath of major storm systems, ephemeral pools capable of supporting floodwater mosquitoes, including Psorophora columbiae, can be too abundant to manage with point-source treatments alone (Breidenbaugh et al. 2008). Hurricanes, excessive rain, or an otherwise unexpected large-scale outbreak of floodwater mosquitoes can be quickly remediated with area-wide intervention using tools such as aerial adulticiding (Breidenbaugh et al. 2008).
In the event that larvicide and adulticide tools are not an option, there are personal protection measures that can help reduce contact with biting mosquitoes. Mass collection traps, many designs of which are available to consumers, have been demonstrated to catch large numbers of Psorophora columbiae when used with appropriate attractants (Allen et al. 2009). In order for trap-and-removal strategies to work, the homeowner must take care to place the trap somewhere outside of normal activity, preferably along harborage such as bushes and other vegetation (Allen et al. 2009). Otherwise, the attractiveness of humans or animals will overpower the trap, leading to aggregation of biting mosquitoes near active areas. Topical skin repellents can reduce biting contact with Psorophora columbiae when products are applied correctly (Qualls et al. 2011). As an alternative, stationary tools or portable emanators that deliver spatial repellents can prevent Psorophora columbiae bites through a combination of repellency (Dame et al. 2014) and mortality (Bibbs et al. 2015) when mosquitoes fail to escape the area.
Selected References
Allen RA, Lewis CN, Meish MV. 2010. Residual efficacy of three spinosad formulations against Psorophora columbiae larvae in small rice plots. Journal of the American Mosquito Control Association, 26: 116–118. DOI: 10.2987/09-0014.1
Allen RA, Lewis CN, Meish MV, Dame DA. 2009. Comparison of three residential mosquito traps against riceland mosquitoes in southeast Arkansas. Journal of the American Mosquito Control Association, 25: 110–112.DOI: 10.2987/08-5805.1
Barr AR, Barr S. 1969. Mosquito Studies (Diptera: Culicidae)—XIII, Pupae of the genus Psorophora in North America and Puerto Rico. Contributions of the American Entomlogical Institution, 4: 1–20.
Bibbs CS, Fulcher AP, Xue RD. 2015. Allethrin based mosquito control device causing knockdown, morbidity, and mortality in four species of field-caught mosquitoes (Diptera: Culicidae). Journal of Medical Entomology, 52: 739–742. DOI: 10.1093/jme/tjv065.
Bickley WE 1984. Notes on the Psorophora confinnis complex. Mosquito Systematics, 16: 162–167.
Bishop F. 1933. Mosquitoes kill livestock. Science 77: 115–116.
Bolling BG, Kennedy JH, Zimmerman EG. 2005. Seasonal dynamics of four potential West Nile vector species in north-central Texas. Journal of Vector Ecology, 30: 186–194.
Botello G, Golladay S, Covich A, Blackmore M. 2013. Immature mosquitoes in agricultural wetlands of the coastal plain of Georgia, U.S.A.: Effects of landscape and environmental habitat characteristics. Ecological Indicators, 34: 304–312.
Breidenbaugh MS, Haagsma KA, Walker WW, Sanders DM. 2008. Post-Hurricane Rita mosquito surveillance and the efficacy of air force aerial applications for mosquito control in east Texas. Journal of the American Mosquito Control Association, 24: 327–330. DOI: 10.2987/5731.1
Britch SC, Linthicum KJ, Wynn WW, Aldridge RL, Walker TW, Farooq M, Dunford JC, Smith VL, Robinson CA, Lothrop BB, Snelling M, Gutierrez A, Wittie J, White G. 2011. Longevity and Efficacy of Bifenthrin Treatment on Desert-Pattern US Military Camouflage Netting Against Mosquitoes in a Hot-Arid Environment. Journal of the American Mosquito Control Association, 27: 272–279.
Burkett-Cadena ND. 2013. Mosquitoes of the southeastern United States. University of Alabama Press.
Carpenter SJ, LaCasse WJ. 1955. Mosquitoes of North America, north of Mexico. University of California Press, Berkeley, CA. 360 pp.
Cleckner HL, Allen TR, Bellows AS. 2011. Remote sensing and modeling of mosquito abundance and habitats in coastal Virginia, USA. Remote Sensing, 3: 2663–2681. DOI:10.3390/rs3122663
Dame DA, Meisch MV, Lewis CN, Kline DL, Clark GG. 2014. Field evaluation of four spatial repellent devices against Arkansas rice-land mosquitoes. Journal of the American Mosquito Control Association, 30: 31–36.
Darsie RF, Ward RA. 2005. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. University Press of Florida, Gainesville, FL. 384 pp.
Edman JD. 1971. Host-feeding patterns of Florida mosquitoes I. Aedes, Anopheles, Coquillettidia, Mansonia and Psorophora. Journal of Medical Entomology, 8: 687–695.
Foster WA, Walker ED. 2018. Mosquitoes (Culicidae), In eds. G. Mullen and L. Durden, Medical and Veterinary Entomology, 3rd edition. Elsevier Academic Press, Cambridge, MA. 270 pp.
Godsey MS, Burkhalter K, Young G, Delorey M, Smith K, Townsend J, Levy C, Mutebi JP. 2012. Entomological investigations during an outbreak of West Nile virus disease in Maricopa county, Arizona, 2010. American Journal of Tropical Medicine and Hygiene, 87: 1125–1131. DOI:10.4269/ajtmh.2012.11-0700
Howell DE, Stiles GW, Moe LH. 1941. The transmission of anaplasmosis by mosquitoes (Culicidae). Journal of the American Veterinary Medicine Association, 99: 107–110.
Kuntz KJ, Olson JK, Rade DJ. 1982. Role of domestic animals as hosts for blood-seeking females of Psorophora columbiae and other mosquito species in Texas rice fields. Mosquito News, 42: 202–210.
Lanzaro GC, Collier TC, Lee Y. 2015. Defining genetic, taxonomic, and geographic boundaries among species of the Psorophora confinnis (Diptera: Culicidae) complex in North and South America. Journal of Medical Entomology, 52: 907–917. DOI: 10.1093/jme/tjv084
Ledesma N, Harrington L. 2012. Mosquito vectors of dog heartworm in the United States: vector status and factors influencing transmission efficacy. Topics in Companion Animal Medicine, 26: 178–185.
McKay T, Bianco T, Rhodes L, Barnett S. 2013. Prevalence of Dirofilaria immitis (Nematoda: Filarioidea) in mosquitoes from northeast Arkansas, the United States. Journal of Medical Entomology, 50: 871–878. DOI: 10.1603/ME12197
Moncayo AC, Lanzaro G, Kang W, Orozco A, Ulloa A, Arredondo-Jiménez J, Weaver SC. 2008. Vector competence of eastern and western forms of Psorophora columbiae (Diptera: Culicidae) mosquitoes for enzootic and epizootic Venezuelan equine encephalitis virus. American Journal of Tropical Medicine and Hygiene, 78: 413–421.
Nasci RS, McLaughlin RE, Focks D, Billodeaux J. 1990. Effects of topically treating cattle with permethrin on Psorophora columbiae (Diptera: Culicidae) blood feeding in a Southwest Louisiana rice-pasture ecosystem. Journal of Medical Entomology, 27: 1031–1034.
Paras KL, O'Brien VA, Reiskind MH. 2014. Comparison of the vector potential of different mosquito species for the transmission of heartworm, Dirofilaria immitis, in rural and urban areas in and surrounding Stillwater, Oklahoma, U.S.A. Medical and Veterinary Entomology, 28: 60–67. DOI: 10.1111/mve.12069
Peloquin JJ, Olson JK. 1985. Observations on male swarms of Psorophora columbiae in Texas ricelands. Journal of the American Mosquito Control Association, 1: 482–488.
Pitzer JB, Byford RL, Vuong HB, Steiner RL, Creamer RJ, Caccamise DF. 2009. Potential vectors of West Nile virus in semiarid environment: Doña Ana county, New Mexico. Journal of Medical Entomology, 46: 1474–1482.
Qualls WA, Mullen GR. 2006. Larval survey of tire-breeding mosquitoes in Alabama. Journal of the American Mosquito Control Association, 22: 601–608. DOI: 10.2987/8756-971X(2006)22[601:LSOTMI]2.0.CO;2
Qualls WA, Xue RD, Holt A, Smith ML, Moeller JJ. 2011. Field evaluation of commercial repellents against floodwater mosquito Psorophora columbiae (Ditera: Culicidae) in St. Johns County, Florida. Journal of Medical Entomology, 48: 1247–1249. DOI: 10.1603/ME11072
Ruiz-Garcia M, Bello F, Ramirez D, Alvarez D. 2006. Genetic structure of the genera Psorophora (Diptera: Culicidae) in Columbian and North American populations using isoenzymes and ITS2 sequences. Russian Journal of Genetics, 42: 752–765. DOI: 10.1134/S102279540607009X
Turrell MJ, Britch SC, Aldridge RL, Xue RD, Smith ML, Cohnstaedt LW, Linthicum KJ. 2015. Potential for Psorophora columbiae and Psorophora ciliata mosquitoes (Diptera: Culicidae) to transmit Rift Valley fever virus. Journal of Medical Entomology, 52: 1111–1116. DOI: 10.1093/jme/tjv093
Unlu I, Kramer WL, Roy AF, Foil LD. 2010. Detection of West Nile virus RNA in mosquitoes and identification of mosquito blood meals collected at alligator farms in Louisiana. Journal of Medical Entomology, 47: 625–633. DOI: 10.1603/ME09087
Wagner RL, Kirby JS, Grogan WL. 2007. Mosquitoes associated with U. S. Department of Agriculture managed wetlands on Maryland's Delmarva peninsula. Journal of the American Mosquito Control Association, 23: 346–350. DOI: 10.2987/8756-971X(2007)23[346:MAWUDO]2.0.CO;2