The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Ring nematodes (Mesocriconema, Criconemoides, Criconemella, etc.) in the family Criconematidae make up a group of nematodes that feed on a wide range of plant hosts. They live in the soil and feed on plant roots as ectoparasites (nematode’s body remains outside of the plant). Most species of ring nematodes are not considered as important plant pests. However, the peach ring nematode Mesocriconema xenoplax and peanut ring nematode Mesocriconema ornatum are two species common in Florida that cause damage to certain agriculturally important crops.
Importance
Species of ring nematode are commonly encountered in natural ecosystems, and in horticultural and agricultural production, but only Mesocriconema xenoplax and Mesocriconema ornatum are known to cause economic damage to plants in Florida. Mesocriconema xenoplax and Mesocriconema ornatum are known to parasitize many different kinds of plants. While direct damage to peach by Mesocriconema xenoplax is difficult to demonstrate, it is a critical player in peach trees' predisposal to peach tree short life (PTSL) (Ritchie and Clayton, 1981) (Figure 1). With PTSL, ring nematode infested trees become susceptible to cold injury and diseases, leading to premature death (Nyczepir et al. 1983). Mesocriconema xenoplax has been associated with chlorosis and decline of lawn grasses in Europe (Inácio et al., 2019), and is considered a minor pest of grape, walnut and carnation. The common Florida lawn grass centipede is particularly susceptible to Mesocriconema ornatum (Ratanaworabhan and Smart, 1969), and it is the primary nematode problem and most common cause of decline of centipedegrass in Florida. Mesocroconema ornatum can cause stunting, yellowing and reduced yield of peanut, decline of bermudagrass on golf courses and athletic fields and it is also a minor pest of blueberry.

Credit: A. P. Nyczepir, USDA ARS (retired) used with permission
Description
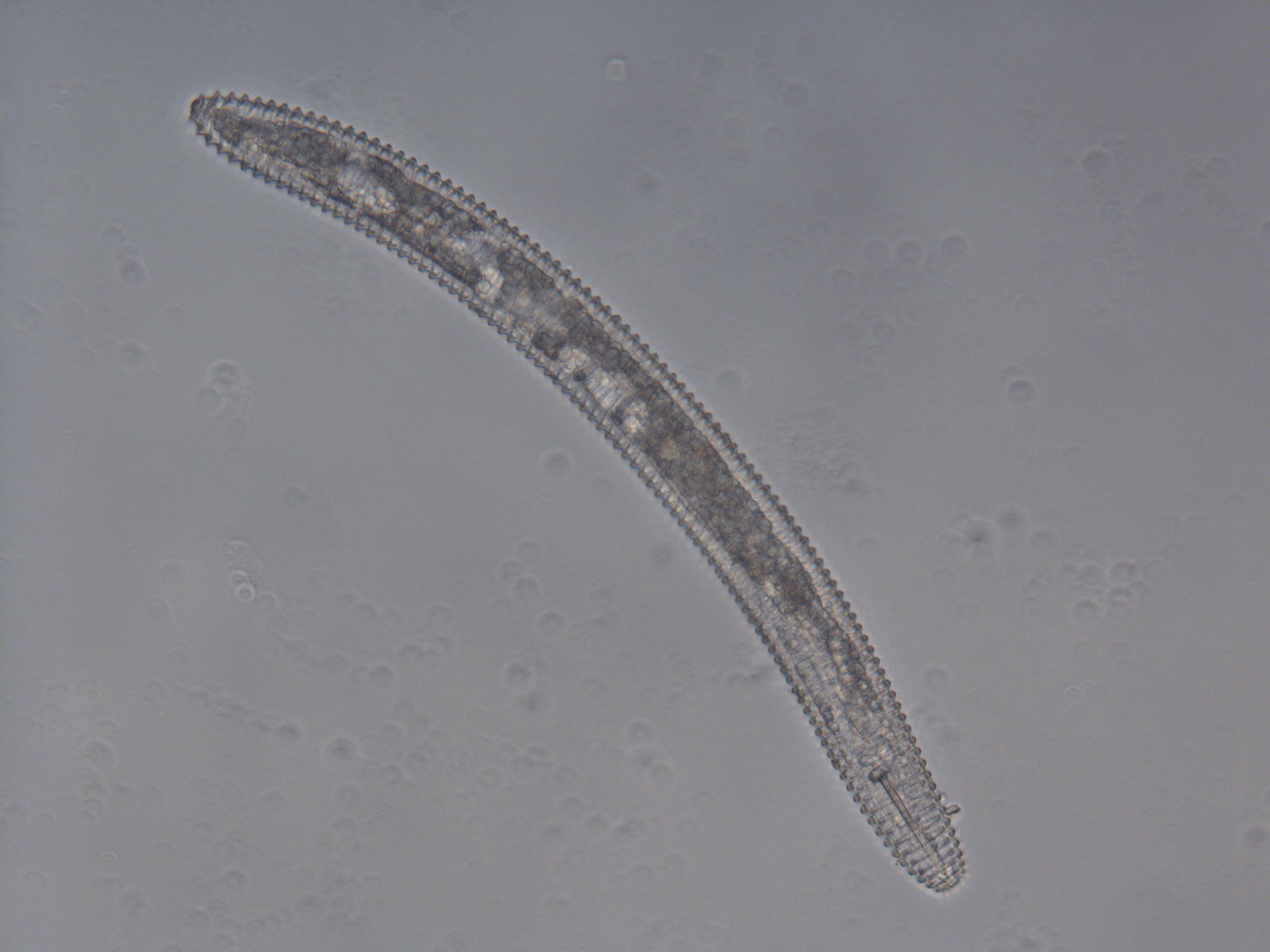
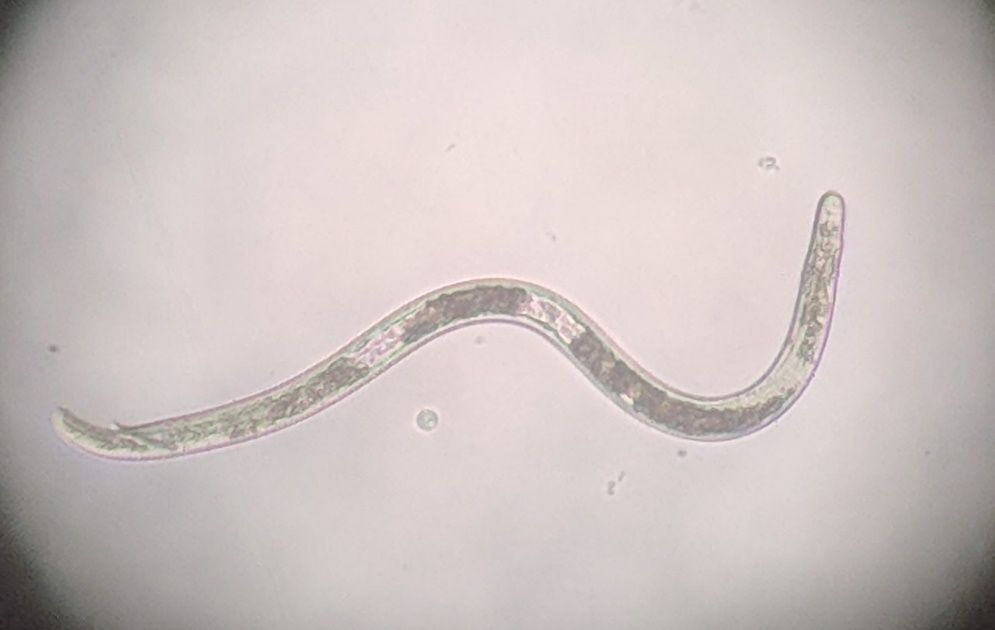
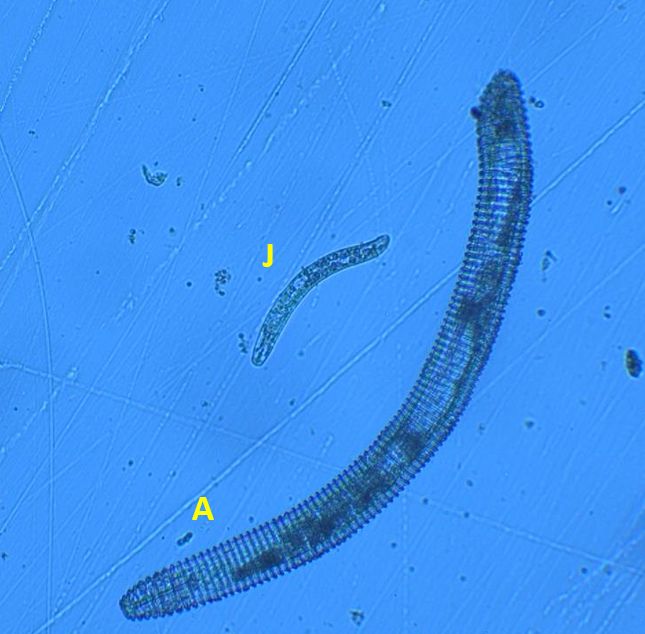
Nematodes in Criconemaidae are characterized by having an amalgamated procorpus (Figure 2). This means that the esophageal lumen, median bulb, and esophageal glands are compressed, and the esophageal glands are greatly reduced. The stylet is well developed and long, and its base is often near, or even within, the median bulb. Mesocriconema are characterized by having thick, rounded, protruding, retrorse cuticular annulations. These annulations make the nematode’s body look like it is made up of “rings” and, hence, the common name ring nematode. The body of Mesocriconema xenoplax and Mesocriconema ornatum is ‘cigar-shaped’; adult females (Figure 3) are generally around 500 µm long and 50 µm wide and their vulva is located posterior, approximately 80-90 percent down the body. Males are rare for both species. When males (Figure 4) are present, they exhibit sexual dimorphism, greatly differing in appearance from the females, have no stylet and apparently do not feed. Juveniles of both species are smaller, and their body shape generally resembles the adult females (Figure 5). Mesocriconema xenoplax and Mesocriconema ornatum are very similar in appearance, have overlapping morphometrics, and are often confused for each other. There are subtle differences in the lip annules (Figure 2), projections in vulval annules, and tail annules (Figure 6), but even trained nematode taxonomists often have a hard time separating them morphologically. Therefore, the easiest way to speciate these ring nematodes is with DNA sequencing.
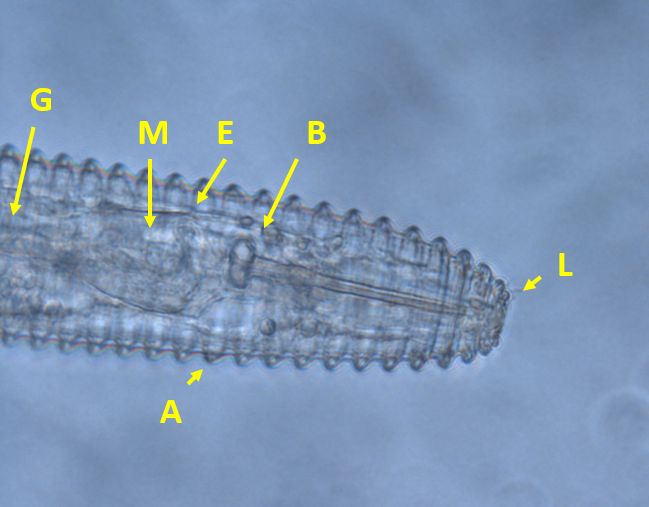
Credit: J. E. Luc, University of Florida
Credit: J. E. Luc, University of Florida
Credit: William T. Crow, UF/IFAS
Credit: William T. Crow, UF/IFAS
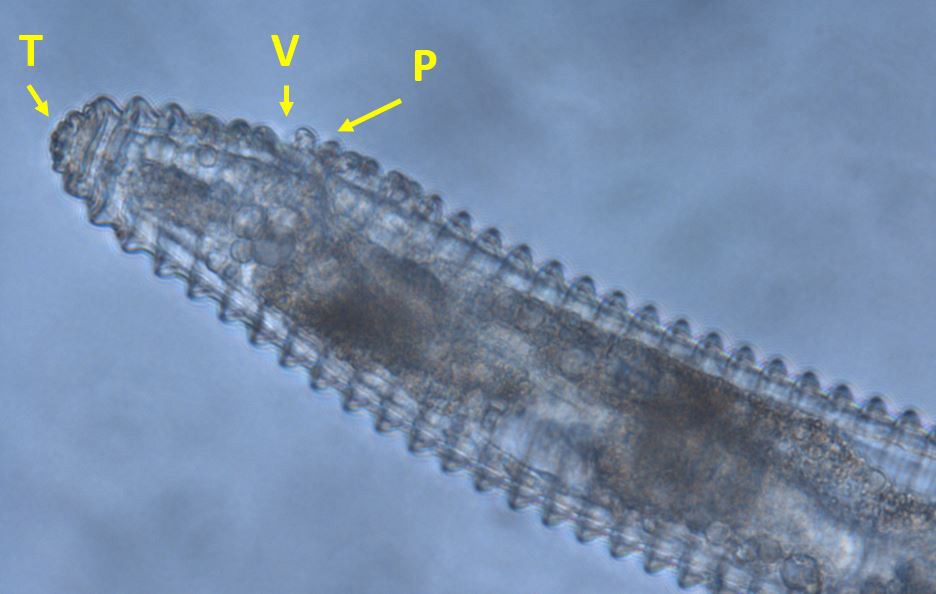
Credit: J. E. Luc, University of Florida
Life Cycle and Biology
Like other nematodes, Mesocriconema xenoplax and Mesocriconema ornatum have four juvenile stages before molting into an adult. The first molt occurs inside the egg and the second-stage juvenile nematode (J2) emerges from the egg. The J2 must feed on live plant cells in order to develop into other life stages. Ring nematodes are ectoparasites, meaning they do not go inside the root with their body to feed. The J2 is attracted to a host root, probes it until a suitable site is located. Once a suitable site is located the nematode inserts its needle-like stylet through the root epidermis and into a live cortical cell. The nematode injects secretions through the stylet and into the cortical cell, causing it to modify into a feeding cell and withdraws nutrients through the stylet to feed. Unlike most other ectoparasitic nematodes that move their feeding site often, Mesocriconema spp. seldom move and remain feeding on a single feeding cell for up to 8 days (Wescott and Hussey, 1992). Upon feeding the J2 molts into a J3 after approximately 3-5 days, and then to a J4 after 5-7 days. During the final molt, the nematode becomes an adult, typically a female. Mesocriconema xenoplax and Mesocriconema ornatum reproduce parthenogenetically without sex. The female ring nematodes lays one egg every two to four days (Thomas, 1959).
Distribution
Mesocriconema xenoplax was first described on grapevines in California in 1952 (Raski 1952). Since then, it has been reported on every continent except Antarctica and throughout the United States on grape, stonefruit, many other woody plants, legumes, different kinds of grasses, forest trees and weeds. It is the most common ring nematode species found on peaches in Florida and Georgia. Mesocriconema ornatum is widely distributed in the United States on peanut and other agronomic plants, vegetable crops, grasses, ornamental plants, forest plants, and weeds. It is the most common plant-parasitic nematode detected from all plants in Florida by the UF Nematode Assay Lab.
Symptoms and Diagnosis
Similar to damage caused by many other plant-parasitic nematodes, symptoms caused by Mesocriconema spp. can be non-descript and difficult to diagnose based on symptoms alone. Mesocriconema xenoplax and Mesocriconema ornatum on turfgrasses are not as virulent as certain other nematode species such as the Grass Root-Knot Nematode Meloidogyne graminis or Sting Nematode Belonolaimus longicaudatus. Still, in high numbers it can cause wilt and decline on most turfgrasses. Centipedgrass is particularly susceptible to Mesocriconema spp. Typically damage appears in irregularly-shaped patches that enlarge very slowly over time (Figure 7). While a minor pest of peanut, Mesocriconema ornatum can reduce pod weight and cause necrotic lesions on pegs, pods, and roots. Mesocriconema ornatum has been implicated as a possible causal agent for blueberry replant disease (Jagdale et al., 2013), a condition where plants being replaced exhibit stunting and slow growth. Mesocriconema xenoplax can result in the predisposal of peach to peach-tree-short-life (Nyczepir et al. 1987). Above-ground symptoms of peach-tree-short-life include collapsed foliage and stunted tree growth (Figure 1) (Beckman and Nyczepir 2005). When cut into, the bark will look brownish, and water soaked. If the peach tree is affected by cold injury the bark will be cracked and separated from the tree trunk (Beckman and Nyczepir 2005. On grape Mesocriconema xenoplax reduced carbohydrate reserves and certain nutrients in the roots and wood, and reduced shade tolerance (Schreiner et al., 2011).
Because symptoms are imprecise for diagnosing ring nematode, diagnosis is primarily based on nematode soil assays performed by a credible nematode diagnostic lab like the UF Nematode Assay Lab. Go to the UF Nematode Assay Lab webpage for instructions on how to collect and submit these kinds of samples. Ring nematodes are among the least mobile plant-parasitic nematodes. Therefore, active extraction methods that depend on nematode movement are not effective for extracting ring nematodes from soil. Passive extraction methods such as centrifugal-flotation or decant-sieving work best for extracting ring nematodes from soil.

Credit: William T. Crow, UF/IFAS
Hosts
Both Mesocriconema xenoplax and Mesocriconema ornatum have extensive host ranges, in fact it is more difficult to identify plants that are non-hosts for these nematodes than to identify hosts. Some of the important crop plants known to be hosts of Mesocriconema xenoplax are: peach, grape, chrysanthemum, walnut, clover, fescue, vetch, ryegrass, curly dock, cowpea. Important hosts of Mesocriconema ornatum are: peanut, all major turfgrasses, blueberry.
Management
Understanding the crop you have, soil type, and what pests can affect your yield are the first steps in developing a management plan. Correct identification of the problem will assist with the management strategy. One way to reduce nematode damage is to replace susceptible plants with those that are more tolerant or resistant. For example; centipedegrass damaged by ring nematodes could be replaced with St. Augustinegrass that is more tolerant. Peach trees declining from peach-tree-short-life can be replaced with peach scion grafted onto Guardian® or another tolerant rootstock (Beckman, 1998). Time of pruning can also mitigate cold injury and reduce wounding and pathogen entry (Beckman and Nyczepir 2005). Breeding programs have developed peach rootstocks and peanut cultivars with resistance to root-knot nematodes, but these do not impart resistance to ring nematodes.
In some cases, use of chemical control is warranted. For fruit production (peach, blueberry, etc.) soil fumigation before planting or replanting can reduce the nematode load and give the new plants a head start on the nematodes. Post-planting nematicides are seldom used on fruit trees. Check with your local Cooperative Extension Service for help determining if any nematicides are currently legal and recommended. In Florida, nematicides are commonly used on peanut, particularly for management of root-knot nematode, and these may provide incidental control of ring nematode at the same time. On golf and high-end sports turf nematicides are commonly used that are effective against Mesocriconema ornatum and other important turfgrass nematodes.
Selected References
Beckman TG. 1998. A guardian angel for peach trees. Agricultural Research October:10-11.
Beckman TG, Nyczepir AP. 2005. Southeastern Peach Grower’s Handbook. 7.4. Peach Tree Short Life, pp. 199-206.
Cordero MA, Robbins RT, Szalanski AL. 2012. Taxonomic and molecular identification of Mesocriconema and Criconemoides species (Nematoda: Criconematidae). Journal of Nematology 44:399–426.
Inácio ML, Rusinque LC, Camacho MJ, Nóbrega F. 2019 First report of Mesocriconema xenoplax (Nematoda: Criconematidae) from turfgrass in Portugal and in Europe. Journal of Nematology 51:e2019-35.
Jagdale GB., Holladay T, Brannen PM, Cline WO, Agudelo P, Nyczepir AP, Noe JP. 2013. Incidence and pathogenicity of plant-parasitic nematodes associated with blueberry (Vaccinium spp.) replant disease in Georgia and North Carolina. Journal of Nematology, 45:92–98.
Nyczepir AP, Reilly CC, Okie WR. 1987. Effects of initial population density of Criconemella xenoplax on reducing sugars, free amino acids, and survival of peach seedlings over time. Journal of Nematology 19:296-303.
Nyczepir AP, Zehr EI, Lewis SA, Harshman DC.1983. Short life of peach trees induced by Criconemella xenoplax. Plant Disease 67:507-508.
Raski DJ. 1952. On the morphology of Criconemoides Taylor 1936, with descriptions of six new species. Proceedings of Helminthological Society of Washington 19:85-99.
Ratanaworbhan, S. and Smart GC. 1969. The ring nematode Criconemoides ornatus, on peach and centipede grass. Journal of Nematology 2:204-208.
Ritchie DF, Clayton CN. 1981. Peach tree short life: A complex of interacting factors. Plant Disease 65:462-469.
Schreiner RP, Pinkerton JN, Zasada IA. 2012. Delayed response to ring nematode (Mesocriconema xenoplax) feeding on grape roots linked to vine carbohydrate reserves and nematode feeding pressure. Soil Biology and Biochemistry 45:89-97.
Thomas HA. 1959. On Criconemoides xenoplax Raski, with special reference to its biology under laboratory conditions. Proceedings of the Helminthological Society of Washington 26:55-59.
Wescott SW, Hussey RS. 1992. Feeding behavior of Criconemella xenoplax in monoxenic cultures. Phytopathology 82:936-940.