The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The mosquito genus Uranotaenia includes about 200 species that occur throughout the temperate and tropical regions of the world. The greatest diversity of Uranotaenia species is found in tropical areas. Uranotaenia sapphirina is one of two members of the genus Uranotaenia occurring in Florida and is native to eastern North America. It is one of the smallest mosquito species in the United States and is striking in appearance, with patches of iridescent blue scales on the sides of the body. This species is unique among mosquitoes in that it specializes on invertebrate annelid hosts, such as earthworms and leeches (Reeves et al. 2018), whereas all other studied blood-feeding mosquito species are specialists of vertebrate hosts. This species is thought to rarely, if ever, feed on humans and other vertebrates and therefore poses little medical or veterinary importance.
Synonomy
Aedes sapphirina Osten Sacken, 1868
Uranotaenia coquilletti Dyar and Knab, 1906
Information gathered from the Integrated Taxonomic Information System and International Commission on Zoological Nomenclature ITIS Report, at www.itis.gov.
Distribution
Three species of Uranotaenia are found in the United States. Uranotaenia sapphirina is the most widespread of these and is one of only two Uranotaenia species occurring in eastern North America (Darsie and Ward 2005). In North America, the geographic distribution of Uranotaenia sapphirina extends from southern Manitoba, Ontario, and Quebec in Canada (Carpenter and LaCasse 1955, Stuart 2007) south through the majority of the eastern United States into Florida, excluding only the northeastern corner of New York, and northern Maine, New Hampshire and Vermont (Darsie and Ward 2005). In Florida, Uranotaenia sapphirina occurs throughout the state. Its range extends westward to the central United States and the upper Midwest with disjunct populations in Colorado and New Mexico (Darsie and Ward 2005), and southeastern Arizona (L. Reeves, unpublished data). Uranotaenia sapphirina also occurs south through eastern and southern Mexico and into the northern Neotropics (Martini 1935, Ortega-Morales et al. 2019, Canto-Mis et al. 2021, Navarrete-Carballo et al. 2021). There are scattered records of this species from Central America (El Salvador), northern South America (Surinam), and the Caribbean (Cuba, Hispaniola, Puerto Rico; Galindo et al. 1954, Broche et al. 2006, WRBU 2021), though these records likely represent Uranotaenia socialis, a closely related and morphologically similar species that replaces Uranotaenia sapphirina in Central and South America (Galindo et al. 1954). In the United States, Uranotaenia sapphirina is morphologically similar to Uranotaenia anhydor and Uranotaenia lowii, the only other Uranotaenia species known from the United States. In some regions west of the Mississippi River in the United States, Uranotaenia sapphirina co-occurs with Uranotaenia anhydor, and in the southeastern United States Uranotaenia sapphirina co-occurs with Uranotaenia lowii (Darsie and Ward 2005).
Description
Adults
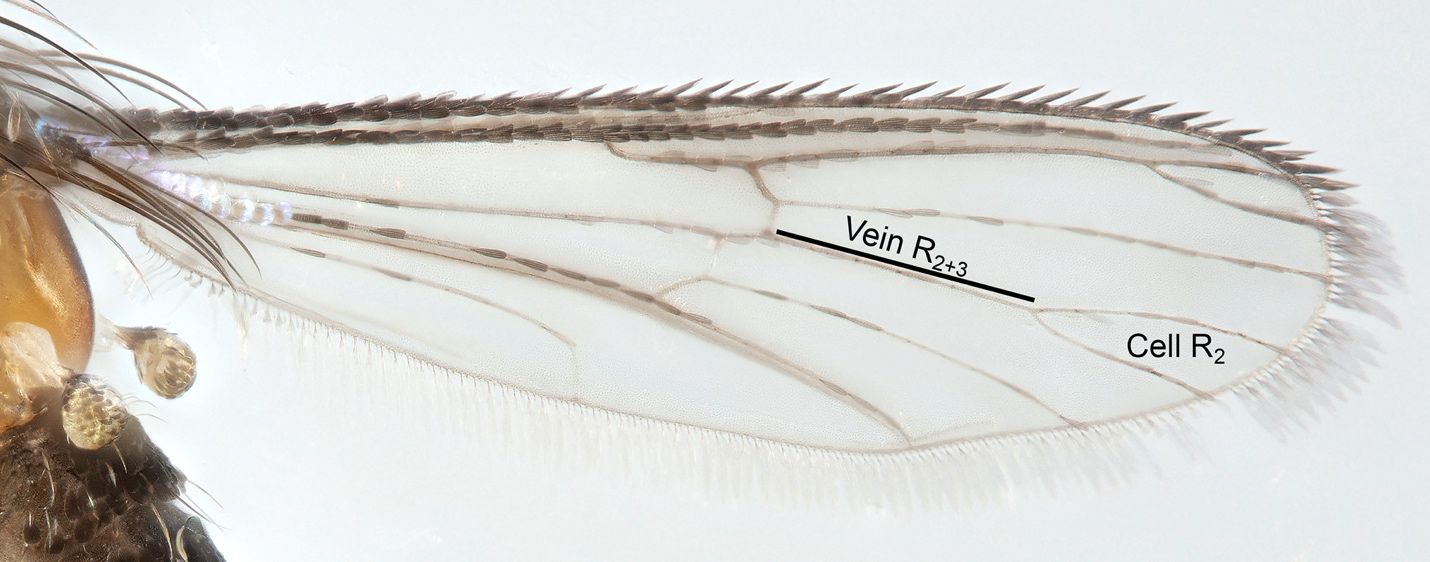
The genus Uranotaenia belongs to the subfamily Culicinae. All species of Uranotaenia are small mosquitoes that can be distinguished from other mosquitoes by wing venation characters (the arrangement of the wing veins; Galindo et al. 1954). The proboscis of the adult male and female is swollen at the end in most species, including all three from the United States, and the palps of both sexes are short. Adult Uranotaenia may be distinguished from other mosquito genera by the following combination of characters: presence of short palps that are less than half the length of the proboscis and an R2 wing cell that is shorter than vein R2+3 (Figure 1) (Darsie and Morris 2003). In the United States, the genus Uranotaenia can be distinguished from other mosquito genera by the presence of patches of iridescent blue scales on the head, thorax, and wings (Figure 2). This unique trait is not shared with any other mosquito genus or species in North America, though a few species within other genera have iridescent purple or green scales (e.g., Toxorhynchites rutilus, species of Psorophora subgenus Janthinosoma, Aedes purpureipes, among others).
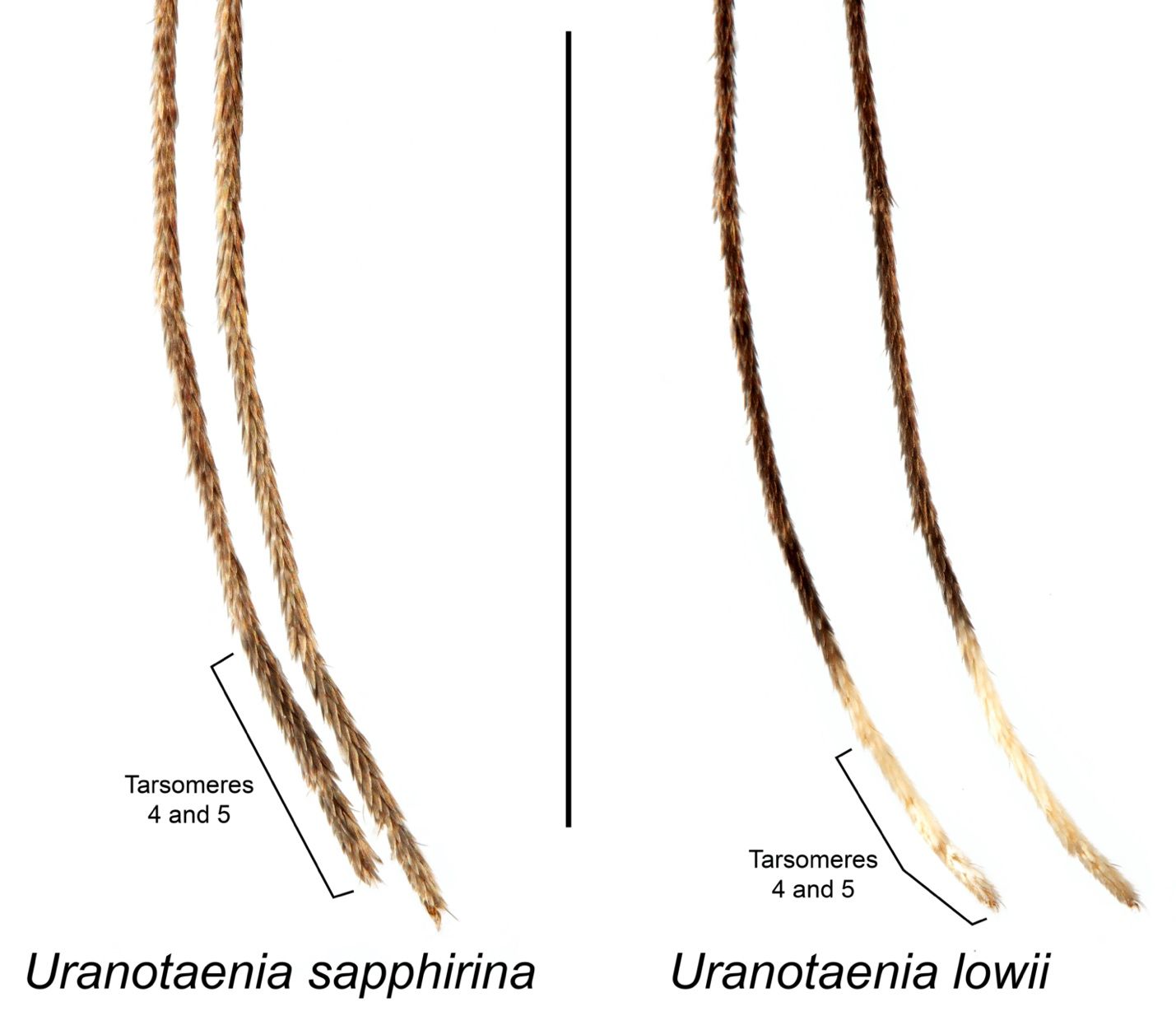
Adult Uranotaenia sapphirina are among the smallest mosquito species in North America. The base color of the body varies from a golden, orange brown to dark brown, almost black. The proboscis of adults of both sexes is swollen at the tip (Figure 2). Uranotaenia sapphirina can be distinguished from the other eastern North American Uranotaenia species, Uranotaenia lowii, by the absence of pale scales on the hind tarsomeres 4 and 5, the last segments of the leg, which are completely dark-scaled in Uranotaenia sapphirina and completely pale-scaled in Uranotaenia lowii (Figures 2 and 3) (Darsie and Morris 2003). They also can be distinguished from Uranotaenia lowii by the absence of iridescent blue scales on the abdomen of Uranotaenia sapphirina, while these scales are present on the abdomen of Uranotaenia lowii (Burkett-Cadena 2013; Figure 2). In some areas west of the Mississippi River in the United States, the range of Uranotaenia sapphirina overlaps with that of Uranotaenia anhydor. These species can be distinguished from each other by the presence of a stripe of iridescent blue scales extending down the center of the scutum (the dorsal surface of the thorax) in Uranotaenia sapphirina, and the absence of this stripe in Uranotaenia anhydor.
Credit: Lawrence Reeves, UF/IFAS

Credit: Lawrence Reeves, UF/IFAS
Credit: Lawrence Reeves, UF/IFAS
Eggs
Like the southern house mosquito, Culex quinquefasciatus, Uranotaenia sapphirina lays rafts of eggs on the surface of the water in vegetated permanent and semi-permanent water bodies (Hinman 1935). The eggs are smaller than those of Culex quinquefasciatus, about 2 mm in diameter, and fewer eggs, 45-50, are laid per raft (Dyar 1901, Dodge 1962). The individual eggs are black and bullet shaped. Eggs in the raft stick together with the pointed side facing upward, and float partially submerged at the surface of the water. Egg rafts are laid on the surface of the water, often among duckweed, Lemna spp. (Figure 4), in permanent or semi-permanent water bodies (Dyar 1901, Hinman 1935).

Credit: Lawrence Reeves, UF/IFAS
Larvae
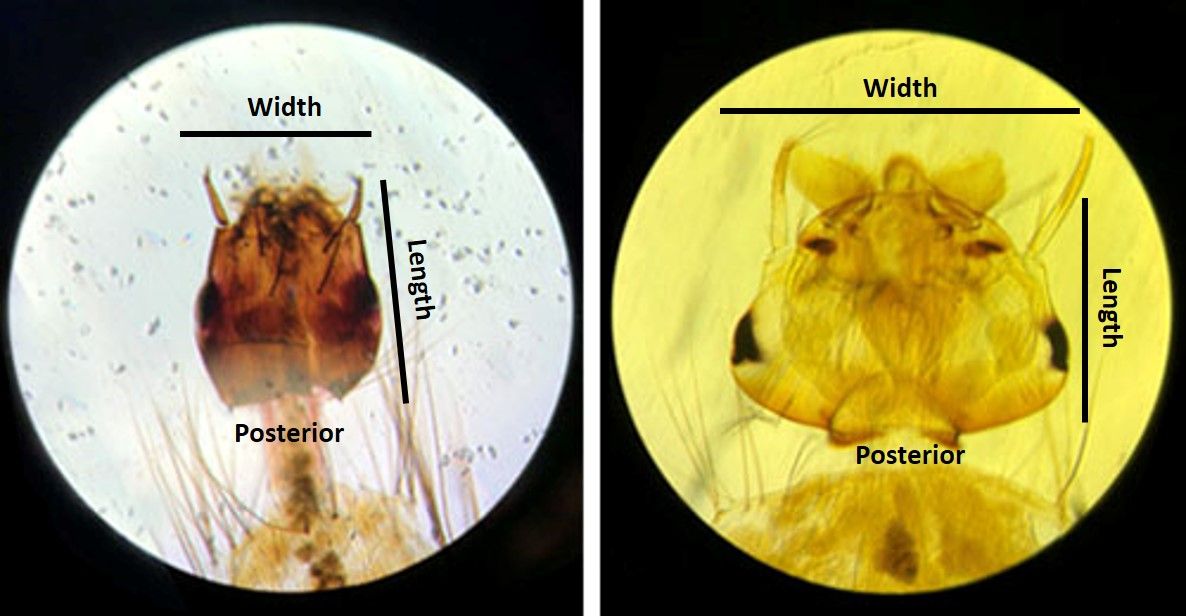
Like other mosquito genera from the subfamily Culicinae, Uranotaenia sapphirina larvae have a siphon, or breathing tube (Figure 5), on the posterior end of the abdomen with a row of spines (pectin spines). Their coloration can vary from pale to dark brown. Uranotaenia larvae can be distinguished from other mosquito genera by the following combination of characters: the presence of a large lateral comb plate, or plate-like structure, located on the posterior end of the abdomen at the base of the siphon, bearing barb-like structures called comb scales and a head of that is longer than it is wide, with four strong, spine-like structures (Figure 6) (Darsie and Morris 2003).
Uranotaenia larvae are small compared to those of other North American mosquito species. Uranotaenia larvae often rest nearly parallel to the surface, which may lead to confusion with Anopheles larvae. At rest, the larvae of Anopheles mosquitoes rest essentially parallel to the surface of the water. Anopheles larvae can be distinguished from those of Uranotaenia by the absence of a siphon (Figure 5) (Stuart 2007). Uranotaenia sapphirina and Uranotaenia lowii larvae are very similar in appearance, but can be distinguished by the number of setae, or hairs, on the thorax (Darsie and Morris 2003).

Credit: Lawrence Reeves, UF/IFAS
Credit: Sarah Maestas, UF/IFAS
Pupae
Like all mosquito species, Uranotaenia sapphirina undergoes a pupal stage between the larval and adult life stages. During the pupal stage, the mosquito does not eat. Mosquito pupae are mobile, and are able to swim throughout their aquatic habitat, propelled by paddles located at the end of the pupal abdomen. They spend much of their time just below the surface of the water but will swim downward if they sense a potential threat. Mosquito pupae have a fused head and thorax, forming the cephalothorax, and an abdomen that forms a characteristic curled shape, with the paddles at the tip (Figure 7). Pupae breath through respiratory trumpets, similar to a snorkel, projecting from the cephalothorax (Figure 7) at the water surface. The pupae of Uranotaenia sapphirina, as the adults, are small in size compared to other mosquito species, resembling those of Culex mosquitoes, though smaller and with unusually long respiratory trumpets (Dyar 1901).

Credit: Lawrence Reeves, UF/IFAS
Life Cycle
Like all mosquitoes, Uranotaenia sapphirina undergoes complete development with four life stages: eggs, larvae, pupae, and adults. Uranotaenia sapphirina eggs are laid in permanent and semi-permanent wetlands (Figure 8), typically characterized by being sunlit and containing floating and emergent vegetation (Joy and Clay 2002, Stuart 2007), and often containing Spirogyra algae (Hinman 1935, Lawlor 1935). Uranotaenia sapphirina larvae often rest underneath leaves of Lemna duckweed or in the shade of rice plants (Hinman 1935, Stark and Meisch 1984). After hatching, larvae develop within these pools throughout the spring and summer months (Hinman 1935), likely feeding from filamentous algae (Dodge 1962). Larvae develop through four larval stages, or instars, shedding the exoskeleton between each. After completing the fourth instar of the larval stage, they molt into pupae, and then eclose into adults. Larvae have been collected as early as January and as late as September. Males and females of most, if not all, mosquitoes require plant-derived sugar for nutrition and energy (Peach and Gries 2019), and Uranotaenia sapphirina adults have been found with plant sugar in their abdomens (Bidlingmayer and Hem 1973). Mating is thought to occur throughout the summer and into the fall. Uranotaenia sapphirina overwinters as adults, with mated females sheltering in dark, humid microhabitats such as caves, hollow trees or similar crevices where they undergo a winter diapause before emerging to lay multiple batches of eggs in the spring (Hinman 1935; Lawlor 1935; Burkett-Cadena et al. 2011). While Uranotaenia sapphirina adults are most often collected near wetlands, they have the potential to disperse substantial distances and adults have been collected over the Gulf of Mexico, 32 km from the shoreline (Sparks et al. 1986).

Credit: Lawrence Reeves, UF/IFAS
Hosts
Uranotaenia sapphirina is the only known mosquito species to specialize on invertebrate host animals, feeding on annelids such as earthworms and leeches (Reeves et al. 2018) (Figure 9). Female Uranotaenia sapphirina have been observed at night congregated in the wet mud at the edge of water sources, where they have been reported to feed on earthworms in the genus Sparganophilus and multiple genera of leeches. While some mosquitoes are autogenous (non-blood-feeding), such as Toxorhynchites spp., feeding only from plant-derived sugars as adults (Steffan and Evenhuis 1981), females of the majority of mosquito species are hematophagous (blood-feeding), using proteins and other nutrients from blood to produce viable eggs. These hematophagous species are known to feed on mammals, birds, reptiles, amphibians, and even fish (Tempelis 1975). Of these, Uranotaenia sapphirina is the only species known to depart from the association with vertebrate hosts of other mosquito species. There is some evidence that Uranotaenia sapphirina will feed on various vertebrate species, although these occurrences may be rare or in error (Irby and Apperson 1988, Cupp et al. 2004). Others found that adult female Uranotaenia sapphirina could not be induced to feed from vertebrate hosts, and prior to the discovery that they feed from invertebrates, were suspected to be autogenous (Headlee 1921).
The linked video demonstrates the feeding habits of Uranotaenia sapphirina (https://www.youtube.com/watch?v=CRY7j0-vtx0) (Credit: Lawrence Reeves, UF/IFAS).

Credit: Lawrence Reeves, UF/IFAS
Medical and Veterinary Importance
Infection with viruses, such as Eastern equine encephalitis and West Nile virus, has been reported from some Uranotaenia sapphirina specimens (Cupp et al. 2003, Andreadis et al. 2004, Armstrong and Andreadis 2010). These pathogens can cause illness and death in both humans and domestic animals. However, the feeding preferences of Uranotaenia sapphirina may preclude significant pathogen transmission risk, as they likely rarely feed on vertebrate hosts. It has been suggested that the source of infection in these mosquitoes may result from feeding on leeches that have in turn taken a blood meal from an infected vertebrate host (Reeves et al. 2018). Vertebrate DNA has been detected in small numbers of Uranotaenia sapphirina during blood meal analysis, and infected blood meals may represent potential sources of infection (Irby and Apperson 1988, Cupp et al. 2004). The host feeding characteristics of this mosquito make it unlikely for an infected individual to transmit pathogens to a human or other susceptible vertebrate animal. Uranotaenia sapphirina has been found infected with a Cypovirus (Shapiro et al. 2005) and a Nucleopolyhedrovirus (Shapiro et al. 2004), though these viruses are not known to infect vertebrates.
Management
The feeding habits of Uranotaenia sapphirina seem to preclude human biting, and it likely only rarely, if ever, feeds on other vertebrate animals. Due to the lack of significance as a vector or pest of medical or veterinary importance, management or control efforts targeting Uranotaenia sapphirina are not warranted.
Selected References
Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. 2004. Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999-2003. Vector-Borne and Zoonotic Diseases 4: 360–378.
Armstrong PM, Andreadis TG. 2010. Eastern equine encephalitis virus in mosquitoes and their role as bridge vectors. Emerging Infectious Diseases 16: 1869–1874.
Bidlingmayer W, Hem DG. 1973. Sugar feeding by Florida mosquitoes. Mosquito News 33: 535–538.
Broche RG. 2006. Culícidos de Cuba (Diptera: Culicidae). Editorial Científico-Técnica, Habana, Cuba.
Burkett-Cadena ND. 2013. Mosquitoes of the Southeastern United States. The University of Alabama Press, Tuscaloosa, AL.
Burkett-Cadena ND, White GS, Eubanks MD, Unnasch TR. 2011. Winter biology of wetland mosquitoes at a focus of eastern equine encephalomyelitis virus transmission in Alabama, USA. Joournal of Medical Entomology 48: 967–973.
Canto-Mis KL, Chan-Chable RJ, Gómez-Rivera AS, López-Sosa XY, González-Acosta C, Correa-Morrales F, Ávila PCM. 2021. Nuevos registros de distribución para Uranotaenia sapphirina (Osten Sacken, 1868) (Diptera: Culicidae) en Quintana Roo, México. Revista Chilena de Entomología 47: 613–617.
Cupp EW, Klingler K, Hassan HK, Viguers LM, Unnasch TR. 2003. Transmission of Eastern equine encephalomyelitis virus in central Alabama. American Journal of Tropical Medicine and Hygiene 68: 495–500.
Cupp EW, Zhang D, Yue X, Cupp MS, Guyer C, Sprenger TR, and Unnasch TR. 2004. Identification of reptilian and amphibian blood meals from mosquitoes in an Eastern equine encephalomyelitis virus focus in central Alabama. American Journal of Tropical Medicine and Hygiene 71: 272–276.
Darsie R, Morris C. 2003. Keys to the adult females and fourth instar larvae of the mosquitoes of Florida (Diptera, Culicidae). Technical Bulletin of the Florida Mosquito Control Association.
Darsie RF, Ward RA. 2005. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. University Press of Florida, Gainesville, FL.
Dodge HR. 1962. Supergeneric groups of mosquitoes. Mosquito News 22: 365–368.
Dyar HG. 1901. The life-history of Uranotaenia sapphirina O.S. Journal of the New York Entomological Society 9: 179–182.
Galindo P, Blanton FS, Peyton EL. 1954. A revision of the Uranotaenia of Panama with notes on other American species of the genus (Diptera: Culicidae). Annals of the Entomological Society of America 47: 107–177.
Headlee TJ. 1921. The mosquitoes of New Jersey and their control. The New Jersey Agricultural Experiment Station Bulletin 348.
Hinman HE. 1935. Biological notes on Uranotaenia spp. in Louisiana (Culicidae, Diptera). Annals of the Entomological Society of America 28: 404–407.
Irby WS, Apperson CS. 1988. Hosts of mosquitoes in the coastal plain of North Carolina, Journal of Medical Entomology 25: 85–93.
Joy JE, Clay JT. 2002. Habitat-use by larval mosquitoes in West Virginia. The American Midland Naturalist 148: 363–375.
Martini E. 1935. Los Mosquitos de México. Departamento de Salubridad Pública. Boletines Técnicos. Serie A: Entomología Médica y Parasitología. No. 1.
Lawlor WK. 1935. Hibernation of Uranotaenia sapphirina (Osten Sacken) (Diptera: Culicidae). Bulletin of the Brooklyn Entomological Society 30: 14.
Navarrete-Carballo J, Chan-Espinoza D, Huerta H, Trujillo-Peña E, López-Platas J, Vivas-Pérez D, Damasco-Córdova K, Medina-Barreiro, Delfín-González H, Manrique-Saide P, Martin-Park A. 2021. Diversity of Culicidae and Tabanidae (Diptera) and new record of Uranotaenia sapphirina from the archaeological site of X’cambó, Yucatan, Mexico. International Journal of Tropical Insect Science 4: 1355–1363.
Ortega-Morales A, Zavortink T, Huerta-Jiménez H, Ibáñez-Bernal S, Siller-Rodríguez S. 2019. The mosquitoes (Diptera: Culicidae) of Hidalgo state, Mexico. Acta Tropica 189: 94–103.
Peach DAH, Gries G. 2019. Mosquito phytophagy – sources exploited, ecological function, and evolutionary transition to haematophagy. Entomologia Experimentalis et Applicata 168: 120–136.
Reeves LE, Holderman CJ, Blosser EM, Gillett-Kaufman JL, Kawahara AY, Kaufman PE, Burkett-Cadena ND. 2018. Identification of Uranotaenia sapphirina as a specialist of annelids broadens known mosquito host use patterns. Communications Biology 1: 92.
Shapiro AM, Becnel JJ, White SE. 2004. A nucleopolyhedrovirus from Uranotaenia sapphirina (Diptera: Culicidae). Journal of Invertebrate Pathology 86: 96–103.
Shapiro AM, Green T, Rao S, White S, Carner G, Mertens PPC, Becnel JJ. 2005. Morphological and molecular characterization of a Cypovirus (Reoviridae) from the mosquito Uranotaenia sapphirina (Diptera: Culicidae). Journal of Virology 79: 9430–9438.
Stark PM, Meisch MV. 1984. Distribution and age composition of mosquito species (Diptera: Culicidae) inhabiting commercial rice fields in southeast Arkansas. Environmental Entomology 13: 1561–1565.
Steffan WA, Evenhuis NL. 1981. Biology of Toxorhynchites. Annual Review of Entomology 26: 159–181.
Sparks AN, Jackson RD, Carpenter JE, Muller RA. 1986. Insects captured in light traps in the Gulf of Mexico. Annals of the Entomological Society of America 70: 132–139.
Stuart TDT. 2007. A new record for Uranotaenia sapphirina (Diptera: Culicidae) in Manitoba, Canada. Proceedings of the Entomological Society of Manitoba 63: 5–7.
Tempelis CH. 1975. Host-feeding patterns of mosquitoes, with a review of advances in analysis of blood meals by serology. Journal of Medical Entomology 11: 635–653.
WRBU (Walter Reed Biosystematics Unit) (2021) Systematic Catalog of Culicidae. https://www.wrbu.si.edu (accessed 4 November 2021).