The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Anopheles stephensi is an urban malaria vector that is responsible for transmitting human malaria in many countries in Southeast Asia, the Middle East, and the Arabian Peninsula (Sinka et al. 2011). In most parts of the world, malaria is a rural disease, and so Anopheles stephensi is unique among other malaria vectors. This mosquito species has recently expanded its geographic range to the Horn of Africa, posing a potential threat for outbreaks of urban malaria, as well as complicating control and elimination of malaria in Africa (Sinka et al. 2020). Anopheles stephensi is highly competent for transmission of malaria parasites, such as Plasmodium falciparum and Plasmodium vivax that can cause mortality among humans, especially children under the age of five (Weiss et al. 2019). There are three biological forms of Anopheles stephensi, known as type, intermediate, and mysorensis. These forms can be distinguished among each other based on egg morphology (Sweet and Rao 1937, Rutledge et al. 1970, Subbarao et al. 1987). The type and intermediate forms tend to be efficient vectors for malaria, while the mysorensis form tends to be zoophilic (prefer animal blood to human blood), which has limited its role as a transmitter of malaria parasites (Subbarao et al. 1987, Sinka et al. 2011, Thomas et al. 2017). In urban settings, Anopheles stephensi (type) tends to be anthropophilic (prefer to ingest blood meals from humans), endophagic (prefer to feed indoors), endophilic (prefer to inhabit/rest indoors), and their juvenile stages can be found in artificial containers (e.g., water barrels, wells, fountains, cisterns). In contrast, in rural settings, Anopheles stephensi (mysorensis) is considered to be zoophilic, exophilic (prefer to inhabit/rest outdoors), and their juvenile stages can be found in irrigation canals, stream pools, and stream margins (Wilkerson et al. 2021).
Synonymy
Anopheles mysorensis Sweet & Rao, 1937 (Wilkerson et al. 2021)
Anopheles folquei de Mello, 1918 (Wilkerson et al. 2021)
Neocellia intermedia Rothwell, 1907 (Wilkerson et al. 2021)
Anopheles metaboles Theobald, 1902 (Wilkerson et al. 2021)
Distribution
This mosquito species is geographically distributed in many counties in Southeast Asia, the Middle East, and the Arabian Peninsula (Figure 1). Recently, it has expanded its geographic range into East Africa, particularly into countries located in the Horn of Africa (e.g., Djibouti in 2012, Ethiopia in 2016, Sudan in 2018, and Somalia in 2019) (Sinka et al. 2020, Ahmed et al. 2021, Hamlet et al. 2022) (Figure 1).
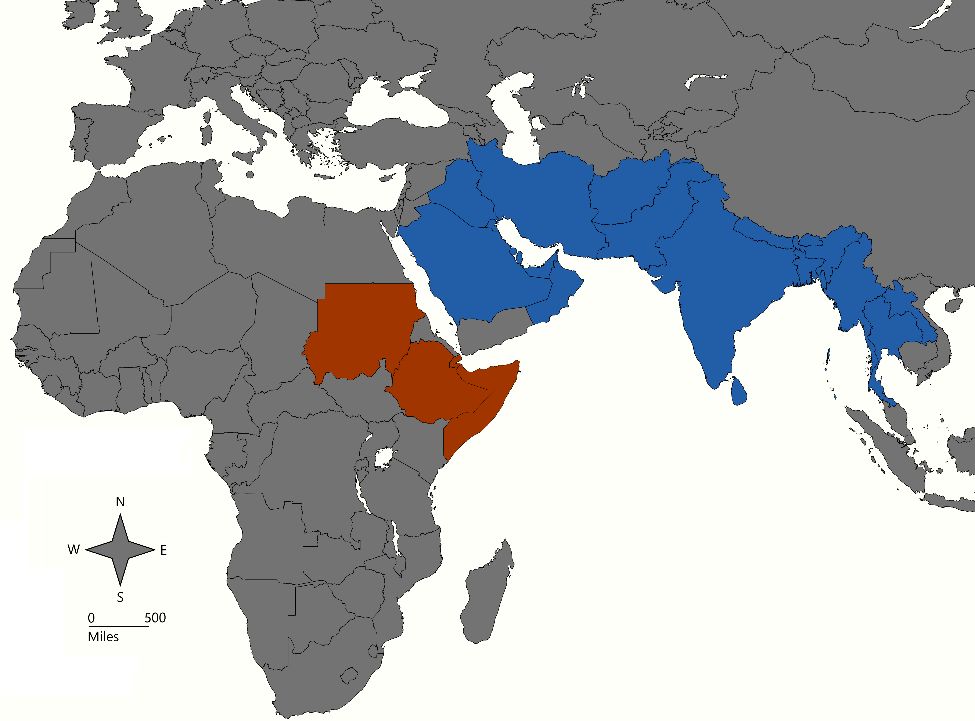
Credit: Abdullah A. Alomar, UF/IFAS
Description
Adult
Anopheles stephensi adult is small to medium in size and light brown to grey in color. The adult body is divided into three principal regions: head, thorax, and abdomen. The head contains the compound eyes, antennae, sensory palps, and a long projecting proboscis used for feeding. The palps of males (club-shaped at the ends) and females (cylindrical in shape) are about as long as the proboscis (Wheelwright et al. 2021). Maxillary palps have pale bands. Apical and subapical pale bands of palpi are equal; palpi speckled (Tyagi et al. 2015). The thorax is the locomotive unit, bearing three pairs of legs and a pair of wings and halteres. The thorax scutum is covered with broad pale scales and setae from dorsal view. Pale spots are present on nearly all veins of wings, and Vein 1A has three dark spots (Wheelwright et al. 2021). The abdomen contains organs for digestion of food and reproduction (egg development in females and testes and accessory glands in males). The segmented sections of the female abdomen can expand noticeably during blood feeding (Figure 2). Abdominal segments II-VII-Te are without dark scale tufts, and V-VII-S are usually with pale scales (Wheelwright et al. 2021). Anopheles mosquitoes can also be identified from other mosquito genera by the resting position: the adult rests with its abdomen pointing away from the body (Foster and Walker 2019).
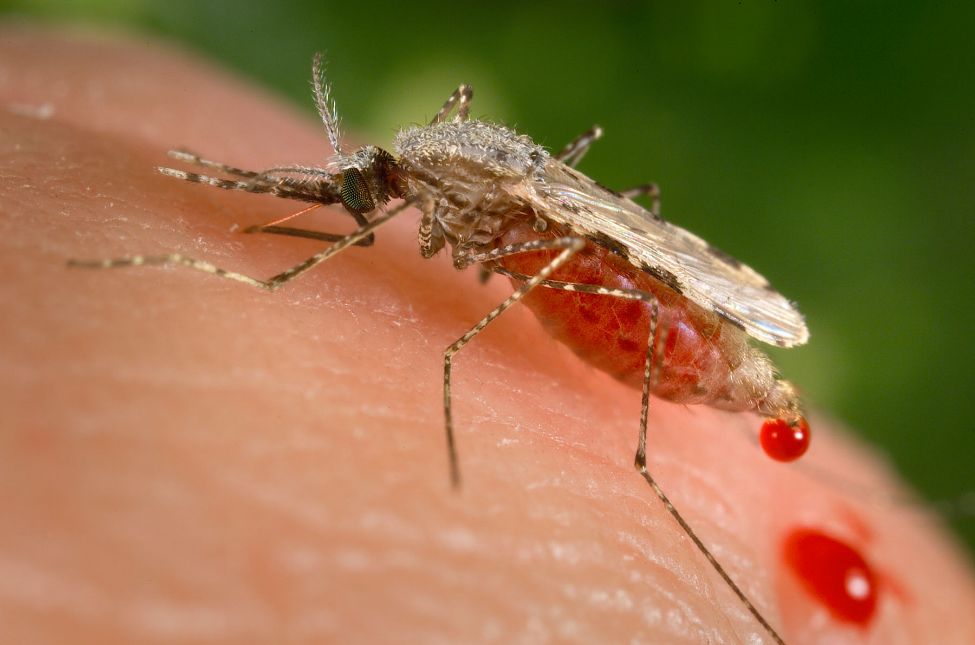
Credit: James Gathany, CDC
Egg
Anopheles stephensi egg is dark brown to black in color and skiff‐shaped in appearance (Figure 3). The ventral egg surface is covered with fine tubercles that are uniform over the whole surface (Malhotra et al. 2000). The egg has floats on either side of the lateral surface (Figure 3). Egg dimensions and the numbers of ridges on the egg float differ between the three biological forms of the species (type, intermediate, mysorensis) (Malhotra et al. 2000, Sinka et al. 2011, Tyagi et al. 2017). The egg has an average length of 473.9 µm and width of 154.6 µm (Tyagi et al. 2017). Females can deposit 50 to 300 eggs individually on the surface of the water after two to three days post blood meal. Eggs are not resistant to desiccation, and they may hatch within two to three days after oviposition. So, unlike Aedes eggs which may remain viable for months before hatching, which is induced by a stimuli from the environment (rainfall which submerges eggs in water and reduced oxygen levels), Anopheles egg biology is more constrained and hatching cannot be delayed, and so they must contend with the current environment to survive.

Credit: Mark Benedict, CDC
Larva
The head of Anopheles stephensi larva is longer than wide, heavily sclerotized, and bearing the antenna, mouthparts, and pairs of setae (Figure 4). In the larval head, seta 1-A is small and unbranched, whereas setae 5,7-C are long and branched (Wheelwright et al. 2021). The abdomen is narrower than the thorax and made up of ten segments. Abdominal seta 1-I has 3-5 branches; setae 9,10-T are both branched; tergal plates on segments IV-VII are small (Tyagi et al. 2015, Wheelwright et al. 2021). The main feature of Anopheles larvae is that the respiratory siphon is absent, and larvae obtain oxygen through a spiracular apparatus located at segment VIII of the abdomen (Burkett-Cadena 2013). Larvae lie parallel to the surface of water and have palmate setae on the dorsal surface of the abdomen that help them cling to the surface tension of the water, where larvae can feed on organic matter and algae present in the water. Larvae pass through four larval instars before developing into pupae. The length of first instar larva is small (~1mm) and increases with larval development, reaching ~ 5 to 8 mm at the final fourth instar.
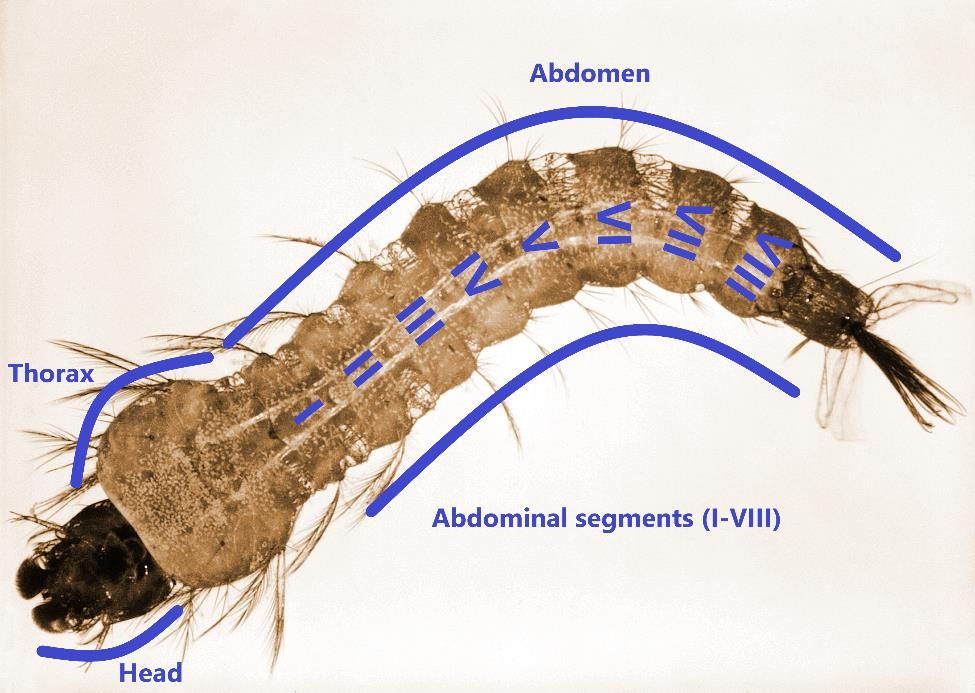
Credit: Harry Weinburgh, CDC
Pupa
Anopheles stephensi pupa, known as a tumbler, is comma-shaped when it is viewed from the side. Pupae are unable to feed and come to the surface frequently to obtain oxygen through the air trumpets (i.e., respiratory tubes) located at the top of the cephalothorax (Figure 5) (Foster and Walker 2019). At the end of the abdomen, there are two broad paddles used for swimming (Figure 5). After a few days, the adult emerges from the pupa and becomes able to fly shortly afterwards.
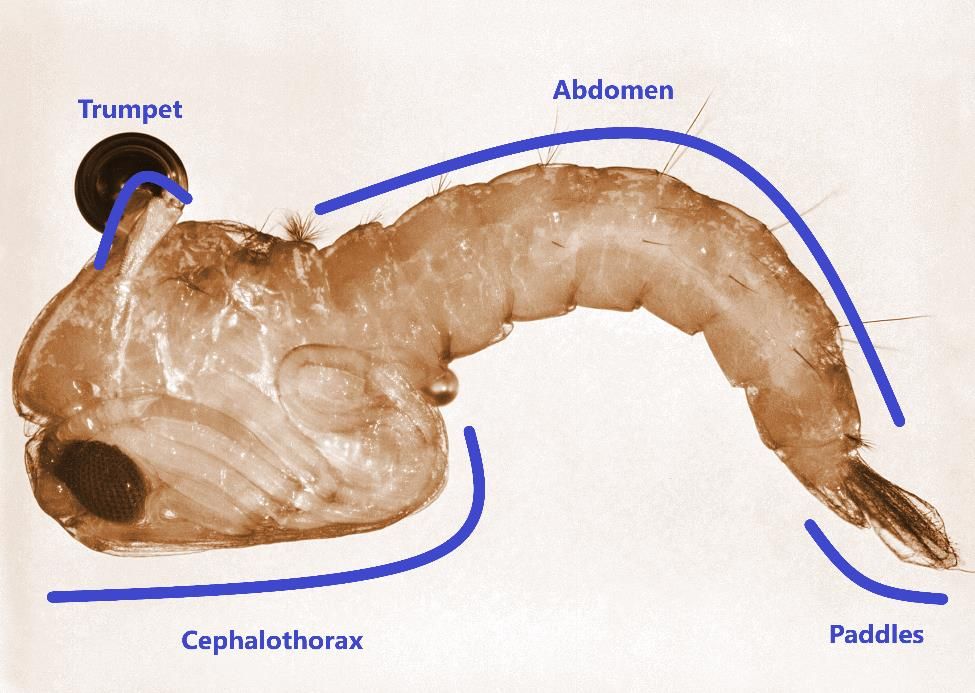
Credit: Harry Weinburgh, CDC
Life History
The life cycle of Anopheles stephensi has two phases, aquatic and terrestrial. The aquatic phase is comprised of the eggs, larvae, and pupae stages, whereas the terrestrial phase is comprised of the adult stage only. Larvae can feed on a variety of organic detritus present in their aquatic habitats. Larvae can develop in a wide range of aquatic habitats, including natural habitats (e.g., marshes, slow flowing rivers, wells, pools, small streams, dips in the ground) and artificial containers (e.g., overhead water tanks, cisterns, hoof prints, domestic wells, borrow pits, empty cans). The larvae of “type” and “intermediate” forms are often found in urban areas in containers with clean water, while the “mysorensis” form is more often found in natural habitats (Sinka et al. 2011). Unlike larvae, pupae are non-feeding, and they undergo further development for a few days (2-3 days) before emerging as adults. Soon after emergence, adult males and females seek sugar sources (e.g., floral nectar and extra-floral plant fluids) to gain sufficient nutrients necessary for energy demanding activities including survival, mating, and flight associated with host seeking (Manda et al. 2007). In order for females to lay eggs, blood meals are required as a source of protein necessary for developing eggs. Blood feeding of adult females often occurs during the nighttime (Sinka et al. 2011). Although the estimation of the adult lifespan varies between studies, adults can live up to one month or longer under optimal conditions (Matthews et al. 2020). While host-feeding preference varies among the three biological forms, in urban areas, blood-meal analyses showed that females tend to feed on humans over other animals, a behavior that accentuates risk of malaria transmission among humans (Sweet and Rao 1937, Sinka et al. 2011). Aestivation, a prolonged dormancy during hot or dry periods, has been proposed as a mechanism for some anophelines to survive the dry season (Dao et al. 2014). In the northern parts of its range, it probably does enter diapause, but may use aestivation in locations with wet/dry seasonality.
Medical Significance
Anopheles stephensi mosquitoes are important vectors of human malaria parasites, particularly Plasmodium falciparum and Plasmodium vivax. This species is regarded as the main malaria vector in urban areas of Southeast Asia (Jiang et al. 2014). Although this mosquito is native to southern and western Asia, it was reported for the first time in the Horn of Africa region, particularly in Djibouti (Faulde et al. 2014), and later in the neighboring country of Ethiopia (Carter et al. 2018). The establishment of this species in continental Africa may pose a significant public health threat for malaria transmission in urban settings, especially since 40% of sub-Saharan Africans live in urban environments (Takken and Lindsay 2019, World Bank 2020). Additional information is needed to determine Anopheles stephensi bionomics in Africa, especially for those characteristics that relate to the ability to transmit parasites (resistance to insecticides, feeding and resting behaviors, susceptibility to parasite infection and transmission) and distribution (climate, habitat preference, seasonality, interactions with other anopheline vectors). Emergence of malaria in African regions is a concern, and there is evidence that Anopheles stephensi was associated with a resurgence of malaria in Djibouti, an area previously considered mostly free of anopheline vectors (Faulde et al. 2014).
Malaria
Malaria is a serious life-threatening disease caused by apicomplexan parasites in the genus Plasmodium and can be spread among humans solely by the bite of an infected Anopheles mosquito (Guerin et al. 2002). There are several species of the Plasmodium genus that affect humans, including Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi. The latter species is unique in that it is the only Plasmodium species that can infect humans from a zoonotic cycle (Moyes et al. 2014). Unlike the other four species, humans are not the primary host for Plasmodium knowlesi. It’s transmission cycle primarily involves non-human primates (monkeys) as the vertebrate host, but there are aspects of its biology that are not well-described, including changes in geographical distribution (Moyes et al. 2014).
Malaria parasites undergo development in both the invertebrate vector (e.g., Anopheles mosquito) and vertebrate host (e.g., human) (Figure 6). When a malaria-infected female Anopheles takes a blood meal, it injects saliva along with the sporozoites into the human, where these sporozoites migrate to infect human’s liver cells, mature into schizonts, and then rupture releasing the merozoites that can infect red blood cells. In the blood, the parasites can replicate inside the red cells and later destroy them, releasing the merozoites into the bloodstream, where they continue to invade red blood cells. These asexual, blood-stage parasites and associated activities are responsible for the malaria symptoms in humans (e.g., fever, rigors, headaches). During blood feeding on Plasmodium-infected hosts, female Anopheles ingest the red blood cells that contain male (microgametocytes) and female (macrogametocytes) Plasmodium gametocytes. These forms of blood stage parasites then mate, grow, and replicate within the gut of female Anopheles. Soon after ingestion by a mosquito, gametocytes in the midgut lumen receive environmental cues (e.g., drop in temperature, presence of xanthurenic acid) that they have left the human host. In response, these parasites rapidly develop into macro- and microgametes (i.e., undergo gametogenesis) and then egress from RBCs. These gametes fuse to form a zygote and, over ~24 hours, each zygote develops into a motile ookinete. To complete the next stage in development, the ookinete must pass through the peritrophic membrane before attaching to and invading a midgut epithelial cell. The ookinete then traverses the cell and transforms into an oocyst once it reaches the basal lamina (a layer of the basement membrane). Inside the basal lamina, each oocyst undergoes multiple rounds of asexual reproduction to produce sporozoites. When mature, oocysts rupture and each oocyst typically releases a few thousand sporozoites into the mosquito’s open circulatory system. A subset of these successfully migrate to and invade the salivary glands. When a female Anopheles feeds on another human, the sporozoites are injected during the blood feeding and the infection cycle inside the human begins again (Baer et al. 2007).
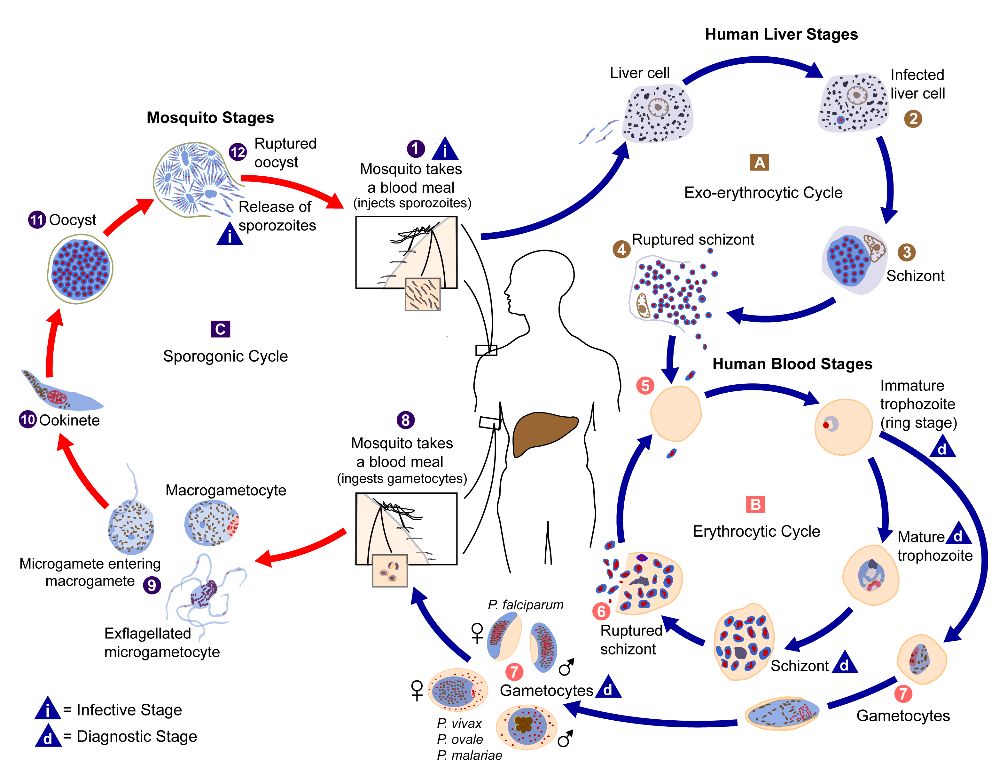
Credit: Alexander J. da Silva, CDC
Management
There are several management practices that can be used to reduce the risk of malaria transmission, including environmental modifications, community education, insecticide-treated bed nets, indoor residual spraying of insecticides, spatial repellents, and biological control. The recent expansion of Anopheles stephensi into East Africa may present challenges for malaria control and warrants reevaluation of ongoing malaria prevention programs, to consider what role this mosquito may play in urban malaria transmission (Benelli and Beier 2017, Sougoufara et al. 2017).
Selected References
Ahmed A, Pignatelli P, Elaagip A, Hamid M, Alrahman O, and Weetman D. 2021. Invasive malaria vector Anopheles stephensi mosquitoes in Sudan, 2016-2018. Emerging Infectious Diseases 27: 2952. https://doi.org/10.3201/eid2711.210040
Baer K, Klotz C, Kappe S, Schnieder T, and Frevert U. 2007. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLOS Pathogens 3: e171. https://doi.org/10.1371/journal.ppat.0030171
Benelli G, Beier JC. 2017. Current vector control challenges in the fight against malaria. Acta Tropica 174: 91-96. https://doi.org/10.1016/j.actatropica.2017.06.028
Burkett-Cadena ND. 2013. Mosquitoes of the southeastern United States. University of Alabama Press.
Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, Ibrahim M, Mohammed S, and Janies D. 2018. First detection of Anopheles stephensi Liston, 1901 (Diptera: Culicidae) in Ethiopia using molecular and morphological approaches. Acta Tropica 188: 180-186. https://doi.org/10.1016/j.actatropica.2018.09.001
Dao A, Yaro AS, Diallo M, Timbiné S, Huestis DL, Kassogué Y, Traoré AI, Sanogo ZL, Samaké D, Lehmann T. 2014. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nature 516: 387-390. https://doi.org/10.1038/nature13987
Faulde MK, Rueda LM, and Khaireh BA. 2014. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Tropica 139: 39-43. https://doi.org/10.1016/j.actatropica.2014.06.016
Foster WA, and Walker ED. 2019. Mosquitoes (Culicidae). In Medical and veterinary entomology. Academic press. https://doi.org/10.1016/B978-0-12-814043-7.00015-7
Guerin PJ, Olliaro P, Nosten F, Druilhe P, Laxminarayan R, Binka F, Kilama WL, Ford N, and White NJ. 2002. Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. The Lancet Infectious Diseases 2: 564-573. https://doi.org/10.1016/S1473-3099(02)00372-9
Hamlet A, Dengela D, Tongren JE, Tadesse FG, Bousema T, Sinka M, Seyoum A, Irish SR, Armistead JS, Churcher T. 2022. The potential impact of Anopheles stephensi establishment on the transmission of Plasmodium falciparum in Ethiopia and prospective control measures. BMC medicine 20: 1-0. https://doi.org/10.1186/s12916-022-02324-1
Ishtiaq F, Swain S, and Kumar SS. 2021. Anopheles stephensi (Asian Malaria Mosquito). Trends in Parasitology 37: 571-572. https://doi.org/10.1016/j.pt.2021.03.009
Jiang X, Peery A, Hall AB, Sharma A, Chen XG, Waterhouse RM, Komissarov A, Riehle MM, Shouche Y, Sharakhova MV, and Lawson D. 2014. Genome analysis of a major urban malaria vector mosquito, Anopheles stephensi. Genome Biology 15: 1-18. https://doi.org/10.1186/s13059-014-0459-2
Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, and Hassanali, A. 2007. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malaria journal 6: 1-11. https://doi.org/10.1186/1475-2875-6-113
Matthews J, Bethel A, Osei G. 2020. An overview of malarial Anopheles mosquito survival estimates in relation to methodology. Parasites & vectors 13: 1-2. https://doi.org/10.1186/s13071-020-04092-4
Malhotra PR, Jatav PC, and Chauhan RS. 2000. Surface morphology of the egg of Anopheles stephensi stephensi sensu stricto (Diptera, Culicidae). Italian Journal of Zoology 67: 147-151. https://doi.org/10.1080/11250000009356307
Moyes CL, Henry AJ, Golding N, Huang Z, Singh B, Baird JK, Newton PN, Huffman M, Duda KA, Drakeley CJ, and Elyazar IR. 2014. Defining the geographical range of the Plasmodium knowlesi reservoir. PLOS neglected tropical diseases, 8: e2780. https://doi.org/10.1371/journal.pntd.0002780
Rutledge LC, Ward RA, and Bickley WE. 1970. Experimental hybridization of geographic strains of Anopheles stephensi (Diptera: Culicidae). Annals of the Entomological Society of America 63:1024-1030. https://doi.org/10.1093/aesa/63.4.1024
Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, and Willis KJ. 2020. A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proceedings of the National Academy of Sciences U.S.A. 117: 24900-24908. https://doi.org/10.1073/pnas.2003976117
Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, Gething PW, Elyazar IR, Kabaria CW, Harbach RE, and Hay SI. 2011. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasites & vectors 4: 1-46. https://doi.org/10.1186/1756-3305-4-89
Sweet WC, and Rao BA. 1937. Races of Anopheles stephensi Liston, 1901. The Indian Medical Gazette 72: 665.
Subbarao SK, Vasantha K, Adak T, Sharma VP, and Curtis CF. 1987. Egg‐float ridge number in Anopheles stephensi: ecological variation and genetic analysis. Medical and veterinary entomology 1: 265-271. https://doi.org/10.1111/j.1365-2915.1987.tb00353.x
Sougoufara S, Doucouré S, Sembéne PMB, Harry M, Sokhna C. 2017. Challenges for malaria control in sub-Saharan Africa: Resistance and behavioral adaptations in Anopheles populations. Journal of vector borne diseases 54: 4-15.
Takken W, and Lindsay S. 2019. Increased threat of urban malaria from Anopheles stephensi mosquitoes, Africa. Emerging Infectious Diseases 25: 1431. https://doi.org/10.3201/eid2507.190301
Thomas S, Ravishankaran S, Justin NJ, Asokan A, Mathai MT, Valecha N, Montgomery J, Thomas MB, Eapen A. 2017. Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malaria journal 16:1-7. https://doi.org/10.1186/s12936-017-1764-5
Tyagi BK, Munirathinam A, and Venkatesh A. 2015. A catalogue of Indian mosquitoes. International Journal of Mosquito Research 2: 50-97.
Tyagi V, Dhiman S, Sharma AK, Srivastava AR, Rabha B, Sukumaran D, and Veer V. 2017. Morphometric and morphological appraisal of the eggs of Anopheles stephensi (Diptera: Culicidae) from India. Journal of Vector Borne Diseases 54: 151.
Weiss DJ, Lucas TC, Nguyen M, Nandi AK, Bisanzio D, Battle KE, Cameron E, Twohig KA, Pfeffer DA, Rozier JA, and Gibson HS. 2019. Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum, 2000-17: a spatial and temporal modelling study. The Lancet 394: 322-331. https://doi.org/10.1016/S0140-6736(19)31097-9
World Bank. 2020. https://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS?locations=ZG. (Accessed 1, December 2021).
Wilkerson RC, Linton YM, and Strickman D. 2021. Anopheles stephensi Liston, 1901.In Mosquitoes of the World. Johns Hopkins University Press.
Wheelwright M, Whittle CR, and Riabinina O. 2021. Olfactory systems across mosquito species. Cell and Tissue Research 383: 75-90. https://doi.org/10.1007/s00441-020-03407-2