The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Pulex irritans Linnaeus is commonly known as the human flea owing to its general association with humans, with human dwellings, and with pets and other animals in close association with humans. In fact, this species has been found associated with human archaeological sediments in Europe and Greenland, underscoring the long-term correlation between humans and this flea (Buckland and Sadler 1989). The human flea is cosmopolitan (Mullen and Durden 2019, Gage 2005, Marquardt et al. 2000), but is found in highest abundance in areas where it can readily find mammalian hosts. Though its relative abundance and overall medical importance is disputed, this flea has been implicated as a potentially important vector of the human pathogens causing plague and murine typhus as well as serving as a potential vector of a tapeworm known to parasitize humans (Eldridge and Edman 2004). This review is intended for a general audience interested in understanding the biology, ecology, and medical importance of the human flea.
It should be noted that this species is not known to have an established distribution that currently includes Florida, but rather is often confused with a closely related species (Pulex simulans). Because of the uncertainty of identification of these species in the literature (Lareshci et al. 2018), along with the cosmopolitan nature of P. irritans, and reported cooccurrence of these species elsewhere (Hass and Wilson 1967) and in the nearby state of Georgia (Durden et al. 2012), this article is relevant to extension entomology in Florida.
Synonymy
Pulex irritans Linnaeus (1758) has several published synonyms (GBIF Secretariat 2019; ITIS Taxonomic Serial No. 189332):
Pulex vulgaris De Geer, 1778
Pulx conepati Cunha, 1914
Pulex irritans bahiensis Cunha, 1914
Pulex irritans fulvus Loff, 1929
Pulex fulvus Loff, 1929
Pulex orientalis Kishida, 1939
Pulex irritans historically was confused in the literature with the closely related Pulex simulans. In fact, Jordan and Rothschild (1908) include Pulex simulans with Pulex irritans (Pulex irritans, var. simulans Baker). However, the species confusion was addressed by Smit (1958), who returned Pulex simulans to species status.
Distribution
The distribution of Pulex irritans is reported to be cosmopolitan (Mullen and Durden 2019, Gage 2005, Marquardt et al. 2000). The Global Biodiversity Information Facility (GBIF 2021) includes over 1,000 results of the species from 1960-2020, with most reports associated with temperate areas. Minimal records are reported in Asia, and there are clusters of records in northern and central Europe. In North America, most recent records are west of the Mississippi River in the United States, though eastern US accounts also exist (Benton 1980), with both Pulex irritans and Pulex simulans reportedly co-occurring geographically (Durden et al. 2012). South America, on the other hand, has few records, though biogeographical studies have suggested that the flea may have originated in Central and South America (Buckland and Sadler 1989), a contention currently under investigation using molecular techniques (Zurita et al. 2019). Because the distribution map is based on georeferenced records, it is unknown if geographic gaps are a result of inhospitable habits or are simply a reflection of limited reporting for this species. For example, records in the GBIF (2021) database (Figure 1B) are largely absent from Africa, though Pulex irritans is known to occur in many areas of the continent (Laudisoit et al. 2007, Eisen et al. 2009), suggesting that the distribution of this flea species is likely more expansive than suggested by reported location records (Figure 1).
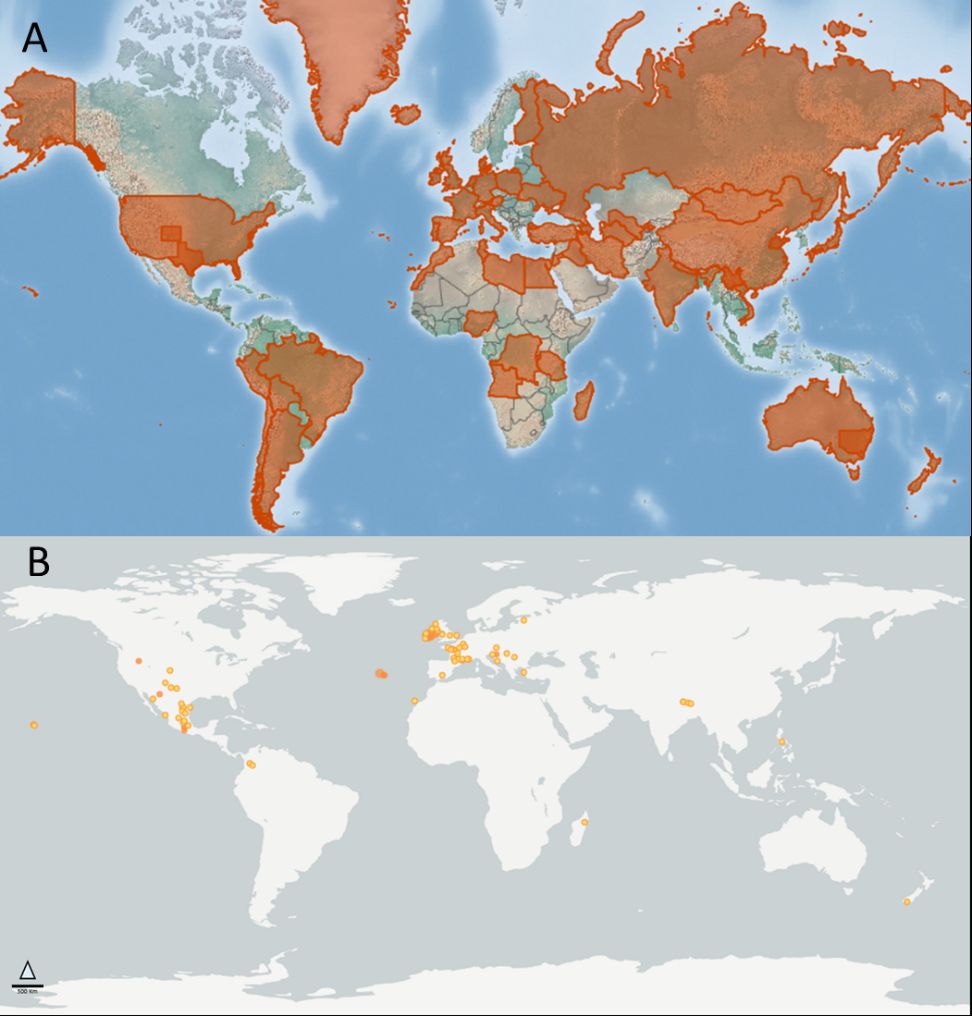
Credit: undefined
Description
Egg
The eggs of Pulex irritans are small and pearl white in color (Wyrwa 2011). The egg is approximately 0.5mm in diameter and morphologically similar from other fleas, and hence is not useful for unique identification of the species.
Larva
The larvae of Pulex irritans are elongated and grub-like in appearance with two small posterior hooks and 13 segments. They are whitish to pale tan in appearance and lack legs. Larvae have few, small setae (bristles) on the body and have a sclerotized (hardened and helmet-like) head, but have no eyes (Wyrwa 2011, Marquardt et al. 2000).
Pupa
The pupae of Pulex irritans are completely inactive and are covered by a loose, silken cocoon. The cocoon itself is initially sticky and allows for environmental debris to adhere to the surface, thus providing some amount of camouflage (Wyrwa 2011, Marquardt et al. 2000).
Adults
Pulex irritans appear as typical, light-brown fleas, with adults averaging 2-3mm in length and males (range 2.0-2.5mm) averaging slightly smaller than females (range 2.5-3.5mm) (Wyrwa 2011, Marquardt et al. 2000). The fleas are laterally compressed (flattened from side-to-side), allowing them to travel through the thick hairs of their hosts more easily. Additional features of this flea that allow for ready identification include a rounded head, absence of a hardened ridge on the mesopleuron (plate over the second pair of legs) to differentiate it from the Oriental rat flea (Xenopsylla cheopis) (Cross, 2021), presence of a single bristle under the eye (ocular bristle), the lack of combs (thickened bristles) below the eyes (the genal comb) and behind the head (the pronotal comb), and a distinctive, comma-shaped spermatheca (sperm storage vessel) in the posterior abdomen that can be observed when a female specimen is cleared and mounted for examination under a light microscope (Figure 2). Males have a noticeable sexual apparatus (the aedeagus) that is readily visible through the examination of a microscopically prepared flea (Figure 3). It is noted that morphological differences between Pulex irritans and the closely related Pulex simulans make differentiating these two species problematic, though Smit (1958) highlights important differences in the male aedeagus. When males of Pulex simulans are detected morphologically, it is then assumed that the females found on the same animal are also of this species, as indicated by Eads et al. (2015). Genetic markers have been investigated to aid in the differentiation of Pulex species as well (Kearl 2013).
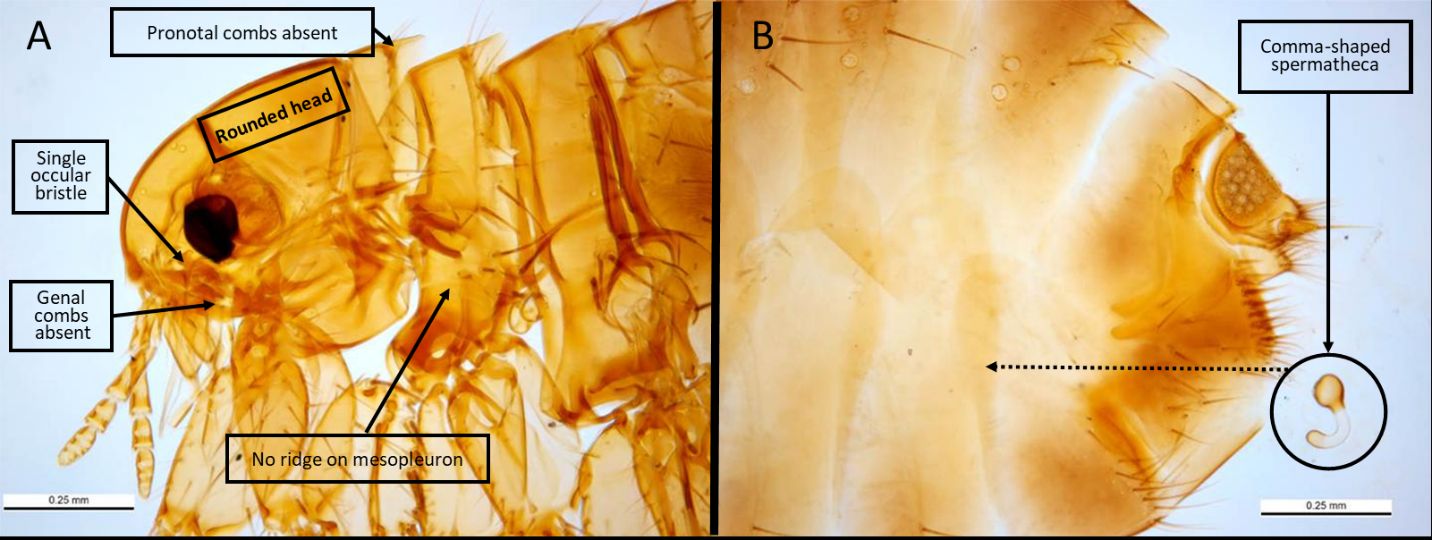
Credit: Ken Walker, Museum Victoria
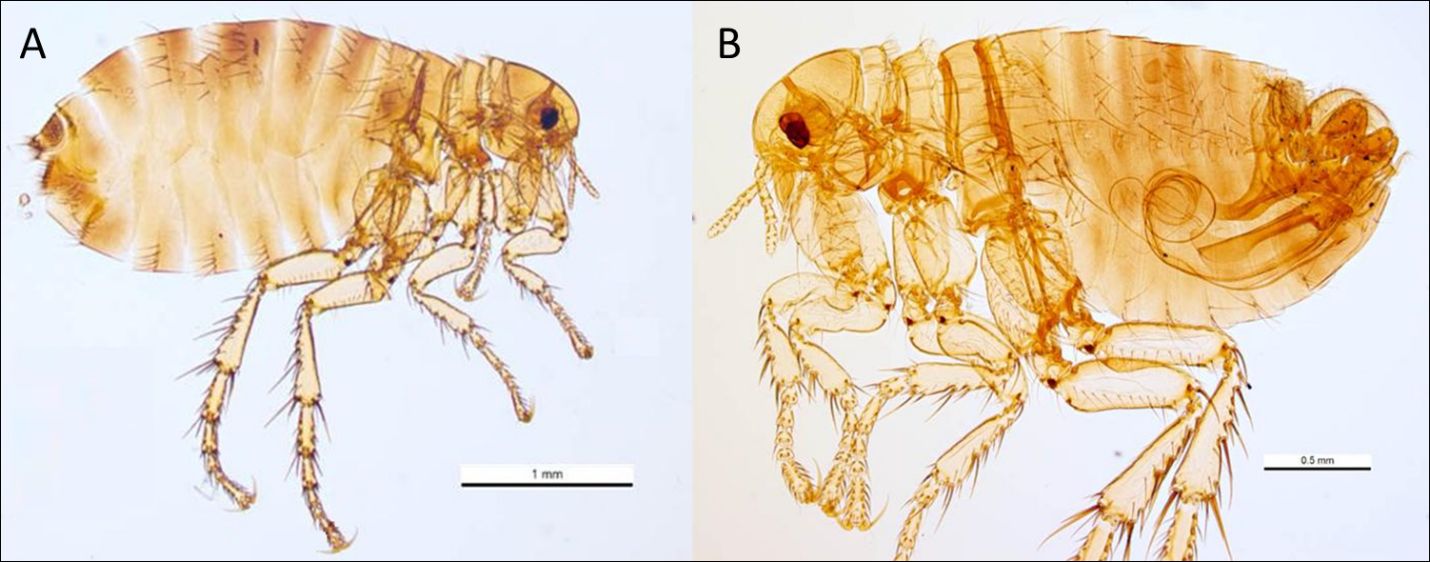
Credit: Ken Walker, Museum Victoria
Life Cycle and Biology
All fleas exhibit holometabolous development (i.e., complete metamorphosis) that consists of an egg stage, larval stage, pupal stage, and then a final transformation to become the adult flea. The developmental cycle of fleas is environmentally influenced, with egg-to-adult development of the human flea averaging 63-77 days (Marquardt et al. 2000). A generalized life cycle diagram is shown in Figure 4.

Credit: Photographs in the public domain and adapted from (2021)
Egg
Eggs of Pulex irritans Linnaeus are laid off the host mammal within the nest environment. Marquardt et al. (2000) suggest an average length of time for egg development to be 7-10 days at 25oC, whereas Wyrwa (2011) suggests 4-6 days. Clearly, there is some relationship of egg developmental time in relation to environmental temperature and humidity, such that warmer temperatures and higher humidity result in shorter developmental time, as suggested for other nest fleas (Cross et al. 2021).
Larva
There are three larval molts in Pulex irritans Linnaeus, with feeding on blood-rich excreta required for growth and development (Marquardt et al. 2000). Larva development averages 11 days (Wyrwa 2011) and ranges from 9-15 days at 25oC but can last as long as 200 days in low-temperature environments (Marquardt et al. 2000).
Pupa
The pupae of Pulex irritans are non-feeding and remain dormant for approximately one week before emerging as adults; however, this time in inversely proportional to temperature and can last for up to 300 days under very cold conditions (Marquardt et al. 2000).
Adult
Pulex irritans has similar adult biology and ecology as many other nest fleas. Reproduction occurs when male fleas make contact with the maxillary palps of the female for identification, after which the male will grasp the female and move into position for insemination to occur. The spermatheca (Figure 2B) receives, maintains, and releases sperm to fertilize eggs (Pascini and Martins 2017; Wyrwa 2011). Marquardt et al. (2000) report as many as 448 eggs produced over a period of 196 days, making fecundity somewhat low, with an average of 2-3 eggs per day. Though longevity of the flea is quite variable, it has been reported that the human flea can live over a year, assuming adequate food is available (Wyrwa 2011).
Pulex irritans are more often found in the nests and burrows of their hosts as opposed to directly on their hosts, unless feeding. In fact, it is this behavior that makes them nuisance species to humans, as they often seek shelter in human homes and outbuildings, particularly in areas where there is close contact between humans and animals, and in which animals may spend time in and around the home (Marquardt et al. 2000).
Medical Significance
Pulex irritans has been implicated as a potential vector of several disease pathogens of medical importance. However, as is common with most flea species, nuisance biting resulting in red papules and dermatitis is quite common in areas with high flea burdens and in locations where humans and animals cohabitate (Mullen et al. 2000, Marquardt et al. 2000). It is the nuisance-biting behavior, however, that can potentially lead to infection with pathogens.
There is long-standing debate on the flea species that have historically played important roles in the transmission of the plague bacterium (Yersinia pestis) that devastated Europe and Asia, and continues to cause local outbreaks in Africa, Asia, South America and the western U.S (Mullen and Durden 2019). Though the Oriental rat flea (Xenopsylla cheopis) is thought to have played a major role in this regard (Cross et al. 2021), the human flea is thought to be a major contributor in some parts of the world (Eldridge and Edman 2004). In fact, the close association of this flea species to humans and human dwellings provides a compelling case for consideration. Eisen et al. (2009) suggest that Pulex irritans should be investigated as a potentially important plague vector, particularly given its reported distribution in endemic plague areas of Africa (e.g., Tanzania, Kenya, and the Democratic Republic of Congo). However, Miarinjara et al. (2021) found in their laboratory studies that Pulex irritans is a relatively poor vector of the plague bacterium in general and is an especially poor vector when the flea feeds on bacteremic human blood (i.e., human blood that is infected with the bacterium). Further studies are needed to confirm any potential role of this flea species in the transmission of the plague bacterium.
Another important consideration is the disease caused by the bacterium Rickettsia typhi. This disease is more commonly known as murine typhus or flea-borne typhus, though Mullen and Durden (2019) also suggest several common names, such as endemic typhus, rat typhus, Mexican typhus, urban typhus, and shop typhus. This bacterium is generally transmitted to humans through flea feces that is infected with the pathogen and can enter the body through scratching it into cuts and abrasions of the skin, and mucous membranes. Because this disease is endemic in many rat species, Pulex irritans can obtain the pathogen through feeding, making it a potentially important vector species of this zoonotic disease. Murine typhus infections result in flu-like symptoms (e.g., fever, chills, nausea, coughing) that may self-resolve, but in some cases may lead to organ failure and prolonged morbidity (CDC 2020).
Finally, many fleas can serve as intermediate hosts to certain helminth species (Mullen and Durden 2019). Pulex irritans has been implicated as an important intermediate host for the double-pored (or dog) tapeworm (Dipylidium caninum). Flea larvae consume egg packets from this parasite when encountered in the environment. When the eggs mature to an infective stage, adult fleas can transmit the tapeworm if they are inadvertently consumed, which happens most often when children accidentally ingest fleas that they contact in the home environment or from pets. The disease condition in humans is called human dipylidiasis (CDC 2019) and is documented in case reports in the medical literature (Narasimham et al. 2013, Cabello et al. 2011, Molina et al. 2003). Most cases of human infection by this helminth are rare and largely asymptomatic but are otherwise readily treated with Praziquantel and other helminth medications (CDC 2019).
Hosts
There are many species that can serve as hosts to Pulex irritans. These include domestic animals such as dogs and cats, rodents, swine, pigs, carnivores, and humans (Mullen and Durden 2009, Marquard et al. 2000). Christodoulopoulos et al. (2006) discusses the importance of dairy goats as hosts in Greece, along with dogs associated with goat herds.
Economic Importance
The ultimate cost of flea-borne diseases is the potential loss of human life. Fortunately, treatments for both plague and murine typhus are readily available through use of antibiotics such as doxycycline (Maurin and Raoult 2017). Should plague become pneumonic or septicemic, healthcare costs can exceed $70,000 for plague (Cross et al. 2021). Additionally, average costs for treatment of murine typhus in the healthcare setting averages nearly $17,000 (Vohra et al. 2018).
Management
Management of Pulex irritans Linnaeus requires control of all life stages of the flea, or there is high risk of reinfestation. Because this flea is found in nests, burrows, and the cracks and crevices of human dwellings, treatment must be designed to penetrate these hard-to-reach areas. In homes with pets, it is important to ensure that dogs and cats are properly treated to reduce their flea burden. Marquardt et al. (2000) suggests vacuuming carpets, cleaning bedding, and cleaning furniture as important management strategies, along with use of flea collars on pets. In areas with urban small mammal pests or in wild populations, host reduction programs through trapping may be useful to reduce flea populations by removing available hosts. According to Boase et al. (2014), there is no single management strategy for fleas, and many attempts at control are only temporary. These authors suggest that residual spraying of insecticides using pyrethroids or insect growth regulators may be useful; however, insecticide resistance in fleas is widespread (Rust 2016). There are some reports of potential flea biocontrol strategies using pathogenic fungi (e.g., Beauveria bassiana) that have shown promise (Eads et al. 2021). Also, certain nematode species (e.g., Steinernema feltiae) have shown success at flea control (Samish et al. 2020). Further research specific to the management of Pulex irritans is needed.
Selected References
Benton HA. 1980. An atlas of the fleas of the eastern United States. Fredonia, New York, 177 p.
Boase C, Kocisova A, Rettich F. 2014. Fleas and flea management. In P. Dhang, Urban Insect Pests: Sustainable Management Strategies. CABI, Oxfordshire, UK. Pp. 86-98. https://doi.org/10.1079/9781780642758.0086
Buckland PC, Sadler JP. 1989. A biogeography of the human flea, Pulex irritans L. (Siphonaptera: Pulicidae). Journal of Biogeography 16: 115-120. https://doi.org/10.2307/2845085
Cabello RR, Ruiz AC, Feregrino RR, Romero LC, Feregrino RR, Zavala JT. 2011. Dipylidium caninum infection. BMJ Case Reports 15: doi: 10.1136/bcr.07.2011.4510. https://doi.org/10.1136/bcr.07.2011.4510
CDC (Centers for Disease Control and Prevention). 2019. Dipylidium caninum. https://www.cdc.gov/dpdx/dipylidium/index.html. (09 November 2021).
CDC (Centers for Disease Control and Prevention). 2020. Flea-borne (murine) typhus. https://www.cdc.gov/typhus/about/murine.html?CDC_AAref_Val=https://www.cdc.gov/typhus/murine/index.html
Christodoulopoulos G, Theodoropoulos G, Kominakis A, Theis JH. 2006. Biological, seasonal and environmental factors associated with Pulex irritans infestation of dairy goats in Greece. Veterinary Parasitology 137: 137-143. https://doi.org/10.1016/j.vetpar.2005.12.012
Cross CL, Williams JL, Lucky A. 2021. Oriental rat flea Xenopsylla cheopis (Rothschild, 1903) (Insecta: Siphonaptera: Pulicidae). EDIS Publication EENY-775. (01 November 2021) https://doi.org/10.32473/edis-IN1330-2021
Durden LA, Wilson N, Eckerlin RP, Baker WW. The flea (Siphonaptera) fauna of Georgia, U.S.A.: hosts, distribution and medical-veterinary importance. Annals of the Carnegie Museum 80:83-113. https://doi.org/10.2992/007.080.0201
Eads DA, Biggins DE, Antolin MF, Long DH, Huyvaert KP, Gage KL. 2015. Prevalence of the generalist flea Pulex simulans on black-tailed prairie dogs (Cynomys ludovicianus) in New Mexico, USA: the importance of considering imperfect detection. Journal of Wildlife Diseases 5:498-502. https://doi.org/10.7589/2014-07-178
Eads DA, Jaronski ST, Biggins DE, Wimsatt J. 2021. Insect pathogenic fungi for biocontrol of plague vector fleas: a review. Journal of Integrated Pest Management 12: 1-10. https://doi.org/10.1093/jipm/pmab028
Eisen RJ, Eisen L, Gage KL. 2007. Studies of the vector competence and efficiency of North American fleas for Yersinia pestis: State of the field and future research needs. Journal of Medical Entomology 46: 737-744. https://doi.org/10.1603/033.046.0403
Eldridge BF, Edman JD. 2004. Medical Entomology: A Textbook on Public Health and Veterinary Problems Caused by Arthropods, Revised edition. Kluwer, Norwell, MA. 659 p.
Gage KL. 2005. Fleas, the Siphonaptera. In Biology of Disease Vectors, WC Marquardt, Ed. Elsevier Academic Press, San Diego, CA. pp. 77-92.
GBIF (Global Biodiversity Information Facility). 2021. Pulex irritans (Linnaeus, 1758). GBIF Backbone Taxonomy. https://www.gbif.org/species/1419547
Hass GE, Wilson N. 1967. Pulex simulans And P. Irritans on dogs in Hawaii (Siphonaptera: Pulicidae). Journal of Medical Entomology 4: 25-30. https://doi.org/10.1093/jmedent/4.1.25
Jordan K, Rothschild NC. 1908. Revision of the non-combed eyed Siphonaptera. Parasitology 1:1-100. https://doi.org/10.1017/S0031182000003280
Kearl CR. 2013. Development of a genetic marker to differentiate between Pulex irritans and Pulex simulans. Undergraduate Thesis, Utah State University. https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=1620&context=honors
Lareshci M, Venzal JM, Nava S, Mangold AJ, Portillo A, Palomar-Urbina AM, Oteo-Revuelta JA. 2018. La pulga humana Pulex irritans (Siphonaptera: Pulicidae) en el noroeste Argentino, una investigación de Bartonella y Rickettsia spp. [The human flea Pulex irritans (Siphonaptera: Pulicidae) in northwestern Argentina, with an investigation of Bartonella and Rickettsia spp.] Review of Mexican Biodiversity 89:375-381. https://doi.org/10.22201/ib.20078706e.2018.2.2392
Laudisoit A, Leirs H, Makundi RH, Van Dongen S, Davis S, Neerinckx S, Deckers J, Libois R. 2007. Plague and the human flea, Tanzania. Emerging Infectious Diseases 13: 687-693. https://doi.org/10.3201/eid1305.061084
Marquardt WC, Demaree RS, Grieve RB. 2000. Fleas and flea-borne Diseases. In Parasitology and Vector Biology, 2nd ed. Harcourt Academic Press, San Diego, CA. pp. 569-582.
Maurin M, Raoult D. 2017. Rickettsia typhi (murine typhus). http://www.antimicrobe.org/r06.asp. (08 November 2021).
Miarinjara A, Bland DM, Belthoff JR, Hinnebusch BJ. 2021. Poor vector competence of the human flea, Pulex irritans, to transmit Yersinia pestis. Parasites & Vectors 14, 317. https://doi.org/10.1186/s13071-021-04805-3
Molina CP, Ogburn J, Adegboyega P. 2003. Infection by Dipylidium caninum in an infant. Archives of Pathology and Laboratory Medicine 127: e157-e159. https://doi.org/10.5858/2003-127-e157-IBDCIA
Mullen GR, Durden LA. 2019. Medical and Veterinary Entomology, 3rd edition. Elsevier, Cambridge, MA. 769 p.
Narasimham MV, Panda P, Mohanty I, Sahu S, Padhi S, Dash M. 2013. Dipylidium caninum infection in a child: a rare case report. Indian Journal of Medical Microbiology 31: 82-84. https://doi.org/10.4103/0255-0857.108738
Pascini TV, Martins GF. 2017. The insect spermatheca: an overview. Zoology 121:56-71. https://doi.org/10.1016/j.zool.2016.12.001
Rust MK. 2016. Insecticide resistance in fleas. Insects 7: 10; doi:10.3390/insects7010010. https://doi.org/10.3390/insects7010010
Samish M, Rot A, Gindin G, Ment D, Behar A, Glazer I. 2020. Biocontrol of the cat flea, Ctenocephalides felis, by entomopathogenic nematodes and fungi. Biological Control 149: 104301. https://doi.org/10.1016/j.biocontrol.2020.104301
Smit FGAM. 1958. A Preliminary Note on the Occurrence of Pulex irritans L. and Pulex simulans Baker in North America. The Journal of Parasitology 44:523-526. https://doi.org/10.2307/3274425
Vohra RF, Walker DH, Blanton LS. 2018. Analysis of health-care charges in murine typhus: Need for improved clinical recognition and diagnostics for acute disease. American Journal of Tropical Medicine and Hygiene 98: 1594-1598. https://doi.org/10.4269/ajtmh.17-0411
Walker, K. 2007. Human flea (Pulex irritans). PaDIL. http://www.padil.gov.au (25 October 2021).
Wyrwa J. 2011. Pulex irritans. Animal Diversity Web. https://animaldiversity.org/accounts/Pulex_irritans/. (01 November 2021).
Zurita A, Callejón R, García-Sánchez ÁM, Urdapilleta M, Lareschi M, Cutillas C. 2019. Origin, evolution, phylogeny and taxonomy of Pulex irritans. Medical and Veterinary Entomology 33: 296-311. https://doi.org/10.1111/mve.12365