Abstract
Culex coronator Dyar and Knab is a highly invasive Neotropical species, first described at the beginning of the 20th century in Trinidad and Tobago (Dyar and Knab 1906). This is an important invasive species in Florida, that needs to be carefully surveilled by mosquito control experts. This publication is structured like those from Walter Reed Biosystematics Unit (WRBU; https://www.wrbu.si.edu/) so that readers can obtain a similar level of detail to that offered through WRBU species pages, which are widely used by anyone interested in mosquito biology, research, and education.
Intended Audience and Purpose
This is the general species information page of Culex coronator for mosquito control professionals, students, scientists, and the general public. This publication is structured like those from Walter Reed Biosystematics Unit (WRBU) so that readers can obtain a similar level of detail offered through WRBU species pages, which are widely used by anyone interested in mosquito biology, research, and education.
Neotropical Region (Nearctic Region) and Part of the United States
[Global Invasive]
Family: Culicidae
Subfamily: Culicinae
Tribe: Culicini
Genus: Culex
Subgenus: Culex
Group: coronator
Brief Species Description
Culex coronator Dyar and Knab is a neotropical species that has been introduced to Florida and the southeastern United States. The species was first described at the beginning of the 20th century in Trinidad and Tobago (Dyar and Knab 1906). Culex coronator is the nominate member of the Culex coronator complex, a group of five cryptic sibling species. The Culex coronator complex includes Culex coronator s.s., Culex camposi Dyar, Culex ousqua Dyar, Culex usquatissimus Dyar, and Culex usquatus Dyar (Bram 1967). All species of the Culex coronator complex have a prominent crown of spines close to the apex. These species are distributed across much of the Neotropics (Bram 1967; Wilke et al. 2020). They have expanded their distribution to most of the Americas where they can occur in sympatry. The sibling species can only be differentiated by characteristics of the male genitalia, while females and immature stages are morphologically indistinguishable (Laurito et al. 2018; Demari-Silva et al. 2017). Recent morphological and molecular evidence suggests that these five species are synonyms of Culex coronator, rather than five distinct species, and they represent a single polymorphic species (Laurito et al. 2018). Culex coronator, in its native range, has been infected with St. Louis encephalitis virus (Anderson et al. 1957) and Venezuelan equine encephalitis virus (Burguete et al. 1973). Recently Zika virus has been detected in the saliva of wild-caught females of this species (Elizondo-Quiroga et al. 2018). However, its role in the transmission of these viruses to humans is unknown.
Type Locality
Trinidad and Tobago
Etymology
Culex from Latin (gnat, mosquito)
coronator, Dyar and Knab 1906: coroner = “crown” in Latin; informal name: “crowned Trinbagonian typical mosquito” (Wilkerson et al. 2021)
coronator subspecies: mooseri Vargas & Martínez Palacios 1954 (Bram 1967); synonymous with coronator (Wilkerson et al. 2021)
camposi Dyar, 1925: campo – “field” in Portuguese; informal name: “campos Ecuadorian typical mosquito” (Wilkerson et al. 2021)
ousqua Dyar, 1918: squalid/foul/filthy in Portuguese; ousia = “lively” in Latin; Informal Name: “Lively Panamanian Typical Mosquito” (Wilkerson et al. 2021)
ousqua subspecies albertoi Anduze 1943; synonymous with ousqua (Wilkerson et al. 2021)
usquatissimus, Dyar 1922: most ancient or old in Portuguese; Informal name: “Large Panamanian Typical Mosquito” (Wilkerson et al. 2021)
usquatus, Dyar 1922: whiskey/aged; Informal Name: “Whiskey Surinamese Typical Mosquito” (Wilkerson et al. 2021)
Type Depository
Global Biodiversity Information Facility (Copenhagen, Denmark). Available at: https://www.gbif.org/species/1653144 (Bánki et al. 2021)
National Ecology Observatory Network (NEON) Biorepository Data Portal (Arizona State University, USA). Available at: https://biorepo.neonscience.org/portal/
National University of Cordoba, Cordoba Argentina
Diagnostic Characters
Adult
Culex coronator is a medium-sized mosquito. The adults are a drab, brownish color (Figure 1). The mesonotum is brown and lacks ornamentation. Much of the body is covered with brown, black, or pale white scales. The presence of obvious pale bands on the hind tarsi of the adult male and female is a notable morphological character, and in Florida, Culex coronator adults can be distinguished from other Culex subgenus Culex species by the presence of these bands and absence of a complete ring of pale scales on the proboscis, although they may have a pale patch on the underside. Culex coronator is somewhat variable in its morphological appearance and coloration. In Florida, the only other Culex subgenus Culex species with apparent bands of pale scales on the hind tarsi are Culex bahamensis and Culex tarsalis. Culex bahamensis occurs only in the far southern Florida Peninsula and the Florida Keys, while Culex tarsalis has been recorded at scattered locations across Florida but is very rare in the state. Both Culex bahamensis and Culex tarsalis have a complete ring of pale scales on the proboscis, and this character distinguishes them from Culex coronator.
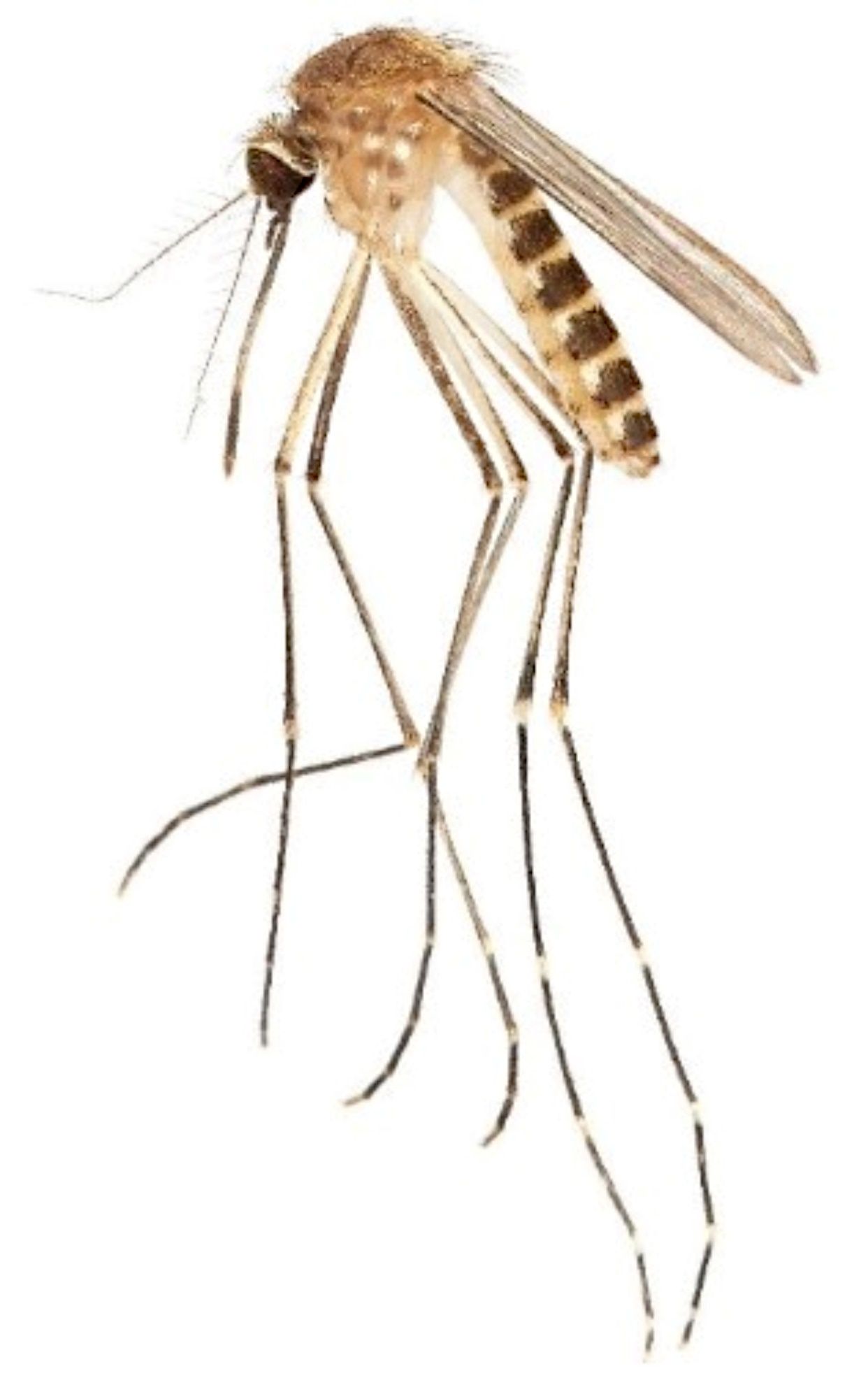
Credit: L. E. Reeves, UF/IFAS
Head
Head with dark erect forked scales dorsally. Occiput with narrow golden scales and bordered with a patch of broad white scales laterally (Figure 2). As in most mosquitoes, the antennae of the male are substantially larger and bushier than those of the female.
Palpus
Palpus without pale scales. The palps of the female are short and entirely dark-scaled (Figure 2).
Proboscis
The proboscis is mostly covered in dark scales with a ventral median area of pale scales that does not form a complete ring; costal and subcostal (Figure 2).

Credit: L. E. Reeves, UF/IFAS
Thorax
The integument of the thorax is brown. The scutum is covered with narrow, golden-brown scales. The mesepimeron and meskatepisternum have small patches of white scales, and, in most specimens, patches of brown integument darker than the background color of the integument above or below the patches of white scales.
Legs
The hind tarsomeres are ringed with distinct white bands (Figure 3). The white band are both basal and apical bands, connecting where the tarsomeres meet.
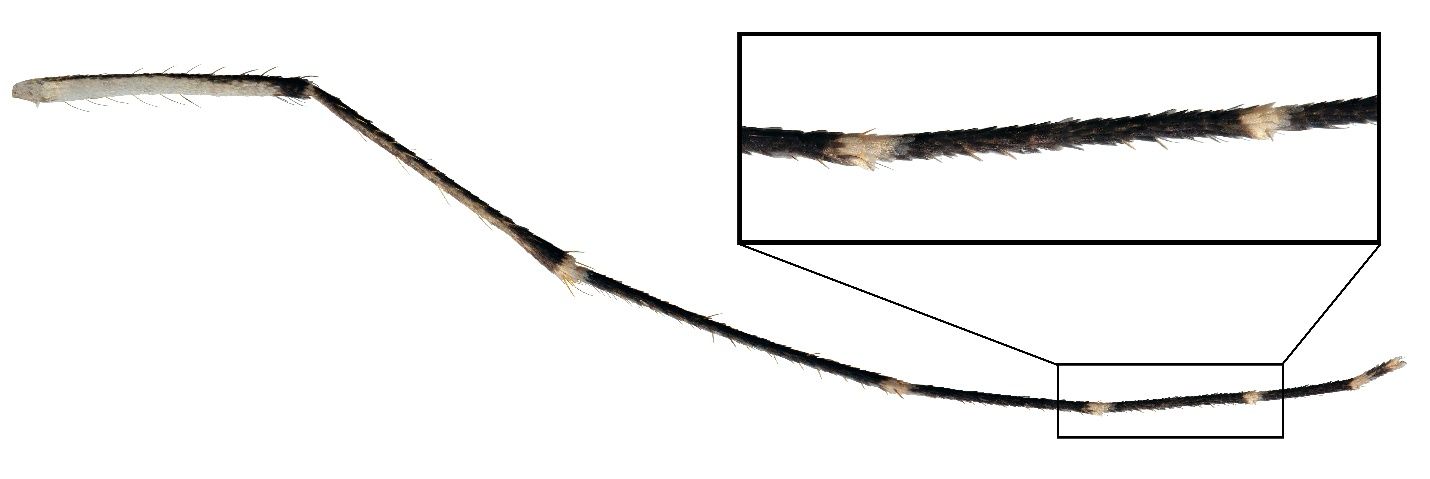
Credit: L. E. Reeves, UF/IFAS
Abdomen
Dorsally, the abdomen is covered in black and white scales (Figure 1). White scales form conspicuous basal bands across each tergite that broaden laterally to form lateral patches of pale scales. The sterna are mostly white, though there is some variability in the extent of patterning with darker scales. The abdomen is black with basal white or pale bands of scales; abdominal sterna without dark triangles.
Wings
The veins of the wings of Culex coronator are covered in narrow, dark scales, typical of species of Culex subgenus Culex; costal and subcostal veins, dark scaled. Wing characteristics are not needed to key out this species (Figure 4).

Credit: A. L. Romero-Weaver, UF/IFAS
Larva
Antennal tuft located in a constriction near the outer third; antennal shaft spiculate basally (Figure 5A).
Head hair, the upper in four or five, the lower in three or four (Figure 5B).
Postclypeal head hair 4 short, single; upper and lower frontal head hairs 5 and 6 long, three or four branched, and barbed; preantennal head hair 7 long, multiple, and barbed.
Mentum with about 15 teeth; the apical tooth is broader and longer than the lateral teeth.
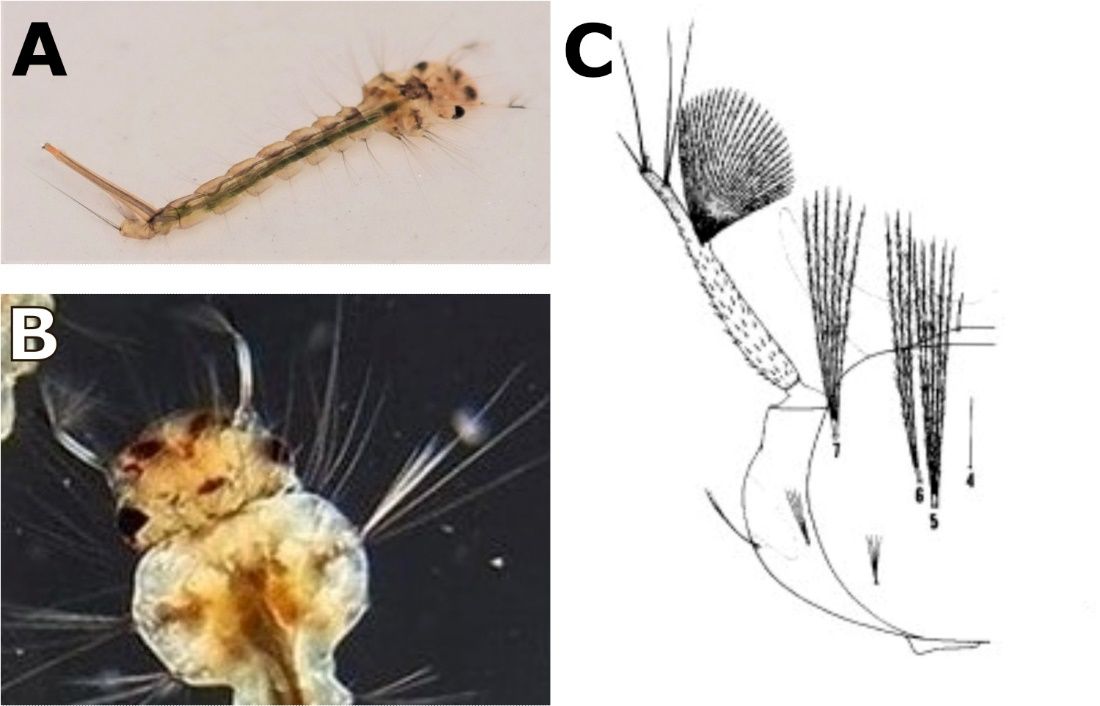
Credit: A) Emmanuel Rodriguez Rojas, National Technological University, Alajuela Costa Rica; B) Walter Ishikawa; C) Carpenter and LaCrosse (1955)
Thorax spiculate. Comb with many scales in a patch; each scale rounded apically and fringed with subequal spinules.
Anal segment spiculate, completely ringed by the saddle, moderate gills.
Siphon long and thin, siphonal index 8.0 to 9.0; four double siphonal tufts inserted on the siphon beyond the pecten; a crown of prominent spines is present before the apex of the siphon of most specimens, with some variability.
Pecten with 8 to 14 teeth on the basal fourth of the siphon; each tooth with two to five coarse barbs on one side. The saddle on segment X with distinct spicules on the posterolateral margins (Figure 6).

Credit: Michael T. Riles, used with permission
Taxonomic Keys
Dyar and Knab 1906
Bram 1967
Clark-Gil and Darsie 1983
Darsie and Ward 2005
Harrison et al 2016
Laurito et al. 2018
Exemplar DNA Sequences
Taxonomy ID 526217
Genbank ID: MF040162.1 (Culex coronator isolate RS10_109 mitochondrion, complete genome; source = Rio Grande do Sul, Brazil)
Bionomics
Immatures
Culex coronator females lay rafts of eggs in diverse natural and artificial microhabitats including swales, roadside ditches, animal water troughs, forest ponds, and rock pools (Dyar and Knab 1906; Goddard et al. 2006; Varando et al. 2012). Artificial water-holding containers including trash cans and car tires have also been reported as larval habitats (Yee et al. 2012)
Adults
Culex coronator can be found in sylvatic, rural, urban, domestic, and suburban habitats. Females of this species are predominantly nocturnal, and blood feed primarily upon large mammals such as white-tailed deer and horses (Almiron and Brewer 1995; Reyes-Villanueva et al. 2006) although they can also blood feed from birds (Mackay et al. 2008). They are low anthropophilic (Carpenter and Lacasse 1955; Consoli and Lourenco-de-Oliviera 1994).
Associated Pathogens
Culex Flavivirus (Miranda et al. 2019)
Ilheus virus (unpublished data reported in Turell et al. 2005)
St Louis encephalitis virus (Aitken et al. 1964; Turell et al. 2005)
Venezuelan equine encephalitis virus (Burguete et al. 1973)
West Nile virus (Mackay 2007; Kelly et al. 2008; Unlu et al. 2010)
Zika virus (Elizondo-Quiroga et al. 2018)
DISTRIBUTION NOTES (Bram 1967; Sames et al. 2021)
Culex coronator is native to Argentina, Belize, Bolivia, Brazil, Colombia, Costa Rica, Chile, Ecuador, El Salvador, Guatemala, Guyana, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, United States, Uruguay, and Venezuela (Figure 7). Between the 1920s and 1970s, Culex coronator was reported from several states in the western and central United States (Arizona, New Mexico, Louisiana, Mississippi, Oklahoma, and Texas). In the early 2000s, the species expanded its United States distribution eastward, and in 2000s and 2010s, it was reported for the first time as far east as Florida and Virginia (Sames et al. 2021).
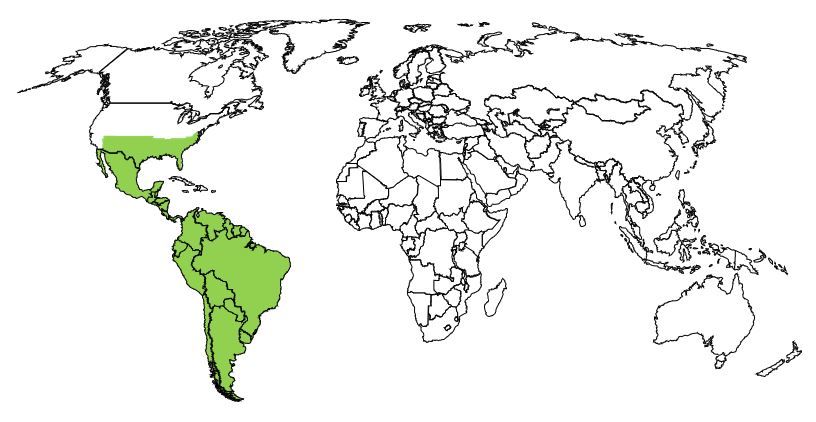
Credit: UF/IFAS
Available GIS Models
None as of December 2021.
Current Synonyms
Culex camposi, Culex ousqua, Culex usquatissimus, Culex usquatus
Funding
We acknowledge funding support of the Southern IPM Center (Project S21-002) as part of USDA National Institute of Food and Agriculture Crop Protection and Pest Management Regional Coordination Program (Agreement No. 2018-70006-28884).
Conflict of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript, or in the decision to publish.
Cited References
Aitken THG, Downs WG, Spence L, Jonkers AH. 1964. St. Louis Encephalitis Virus Isolations in Trinidad, West Indies, 1953–1962. American Journal of Tropical Medicine and Hygiene 13: 450–451. https://doi.org/10.4269/ajtmh.1964.13.450
Almiron WR, Brewer MM. 1995. “Host preferences of Culicidae (Diptera) collected in central Argentina.” Revista de Saúde Pública 29:108–114. https://doi.org/10.1590/S0034-89101995000200004
Anderson CR, Aitken TH, Downs WG, Spence L. 1957. “The isolation of St. Louis virus from Trinidad mosquitoes.” American Journal of Tropical Medicine and Hygiene 6:688–692. https://doi.org/10.4269/ajtmh.1957.6.688
Anduze PJ. 1943. “Estudios de Entomologia Medica en el Estado Merida-Venezuela”. Boletín de Entomología venezolana, 2(4).
Bánki O, Roskov Y, Vandepitte L, DeWalt RE, Remsen D, Schalk P, Orrell T, Keping M, Miller J, Aalbu R, Adlard R, Adriaenssens E, Aedo C, Aescht E, Akkari N, Alonso-Zarazaga MA, Alvarez B, Alvarez F, Anderson G, et al. 2021. “Culex coronator Dyar & Knab, 1906.” in Catalogue of Life Checklist (Version 2021-08-25). Catalogue of Life. https://doi.org/10.48580/d4sg (accessed on 26 October 2021).
Bram R. 1967. “Classification of Culex subgenus Culex (Diptera: Culicidae)”. Proceedings of the United States National Museum 120:1–123. Available at: https://repository.si.edu/bitstream/handle/10088/16935/1/USNMP-120_3557_1967.pdf (accessed on 25 October 2021) https://doi.org/10.5479/si.00963801.120-3557.1
Burguete J, Romero-Acevedo S, Salido RF, Pierce EP. 1973. “Epizootic and epidemic of Venezuelan equine encephalitis in the state of Morelos.” Salud Pública de México 15:231–235.
Carpenter SJ, LaCasse WJ. 1955. “Mosquitoes of North America (North of Mexico).” Berkeley, CA: University of California Press. https://doi.org/10.1525/9780520325098
Clark-Gil S, Jr. Darsie RF. 1983. “The mosquitoes of Guatemala, their identification, distribution and bionomics, with keys to adult females and larvae, in English and Spanish.” Mosquito Systematics 15:151–284. https://www.biodiversitylibrary.org/content/part/JAMCA/MS_V15_N3_P151-284.pdf
Consoli RAGB, Lourenço-de-Oliveira R. 1994. “Principais mosquitos de importância sanitária no Brasil.” Editora Fiocruz, Rio de Janeiro. Brasil Press 97–114. https://doi.org/10.7476/9788575412909
Demari-Silva B, Multini LC, Suesdek L, Oliveira TMP, Sallum MAM, Marrelli MT. “Wing Morphometry and genetic variability between Culex coronator and Culex usquatus (Diptera: Culicidae), two sibling species of the coronator group.” Journal of Medical Entomology 54:901–908. https://doi.org/10.1093/jme/tjx033
Dyar HG, Knab F. 1906. “The larvae of mosquitoes classified as independent organisms.” Journal of the New York Entomological Society 14:169–230.
Dyar HG, Knab F. 1918. “New American mosquitoes”. Insecutor Inscitiae Menstruus, 6(7–9), pp.120–129. https://doi.org/10.5962/bhl.part.14938
Dyar HG. 1922. “Mosquito notes (Diptera, Culicidae)”. Insecutor Inscitiae Menstruus. 10(4–6), 92–99.
Dyar HG. 1925. “Some mosquitoes from Ecuador (Diptera, Culicidae)”. Insecutor Inscitiae Menstruus. 13:27–31.
Elizondo-Quiroga D, Medina-Sánchez A, Sánchez-González JM, Eckert KA, Villalobos-Sánchez E, Navarro-Zúñiga AR, Sánchez-Tejeda G, Correa-Morales F, González-Acosta C, Arias CF, López S, Del Ángel RM, Pando-Robles V, Elizondo-Quiroga AE. 2018. “Zika Virus in Salivary Glands of Five Different Species of Wild-Caught Mosquitoes from Mexico.” Scientific Reports. 8:809–815. https://doi.org/10.1038/s41598-017-18682-3
Goddard J, Varnado WC, Harrison BA. 2006. “Notes on the ecology of Culex coronator Dyar and Knab in Mississippi.” Journal of the American Mosquito Control Association. 22:622–625. https://doi.org/10.2987/8756-971X(2006)22[622:NOTEOC]2.0.CO;2
Harrison BA, Byrd BD, Sither CB, Whitt PB. 2016. “Mosquitoes of the Mid-Atlantic Region: An Identification Guide.” Western Carolina University, Cullowhee, North Carolina. Mosquito and Vector-Borne Infectious Diseases Laboratory Publication. 2016–01. 207 pp.
Kelly R, Mead D, Harrison BA. 2008. “The discovery of Culex coronator Dyar and Knab (Diptera: Culicidae) in Georgia.” Proceedings of the Entomological Society of Washington 110:258–260. https://doi.org/10.4289/0013-8797-110.1.258
Laurito M, Briscoe AG, Almirón WR, Harbach RE. 2018. “Systematics of the Culex coronator complex (Diptera: Culicidae): Morphological and molecular assessment.” Zoological Journal of the Linnean Society 182:735–757. https://doi.org/10.1093/zoolinnean/zlx053
Mackay AJ. 2007. “Detection of West Nile virus activity in male and female mosquitoes, and evaluation of host utilization patterns of mosquitoes, in East Baton Rouge, Louisiana.” [Ph.D. dissertation]. Louisiana State University, Baton Rouge, Louisiana.
Miranda J, Mattar S, Gonzalez M, Hoyos-López R, Aleman A, Aponte J. 2019. “First report of Culex flavivirus infection from Culex coronator (Diptera: Culicidae), Colombia.” Virology Journal 16:1. https://doi.org/10.1186/s12985-018-1108-2
Reyes-Villanueva F, Barrientos-Lozano L, Rodriguez-Pérez MA. 2006. “Feeding pattern of mosquitoes (Diptera: Culicidae) transmitters of the West Nile virus collected from horses and humans in northern Mexico.” Veterinaria México 37:407–415. http://veterinariamexico.unam.mx/index.php/vet/article/view/171
Sames WJ, Mann JG, Kelly R, Evans CL, Varnado WC, Bosworth AB, Noden BH, Ramberg FB, Riles MT, Killingsworth D, Doyle MS, Pitts RJ. 2021. “Distribution of Culex coronator in the USA.” Journal of the American Mosquito Control Association 37:1-9. https://doi.org/10.2987/21-6995.1
Turell MJ, O’Guinn ML, Jones JW, Sardelis MR, Dohm DJ, Watts DM, Fernandez R, Travassos da Rosa A, Guzman H, Tesh R, Rossi CA, Ludwig GV, Mangiafico JA, Kondig J, Wasieloski LP, Jr. Pecor J, Zyzak M, Schoeler G, Mores CN, Calampa C, Lee JS, Klein TA. 2005. “Isolation of viruses from mosquitoes (Diptera: Culicidae) collected in the Amazon Basin region of Peru.” Journal of Medical Entomology 42: 891–898. https://doi.org/10.1093/jmedent/42.5.891
Unlu I, Kramer WL, Roy AF, Foil LD. 2010. “Detection of West Nile virus RNA in mosquitoes and identification of mosquito blood meals collected at alligator farms in Louisiana.” Journal of Medical Entomology 47: 625–633. doi: 10.1603/ME09087. https://doi.org/10.1603/ME09087
Varnado WC, Goddard J, Harrison BA. 2005. “New state record of Culex coronator Dyar and Knab (Diptera: Culicidae) from Mississippi.” Proceedings of the Entomological Society of Washington 107:476–477.
Veggiani Aybar CA, Rossi GC. 2017. “Mosquitoes (Diptera: Culicidae) of the meridian zone of the subtropical mountainous rainforest of Argentina: Update on the fauna and geographical distribution.” Check List 13:2102. https://doi.org/10.15560/13.2.2102
Wilke ABB, Benelli G, Beier JC. 2020. “Beyond frontiers: On invasive alien mosquito species in America and Europe.” PLoS Neglected Tropical Diseases 14:1e0007864. https://doi.org/10.1371/journal.pntd.0007864
Wilkerson RC, Linton YM, Strickman D. 2021. “Mosquitoes of the World (Vol. 1)”. Johns Hopkins University Press. Baltimore, MD. ISBN-13: 978-1421438146
Yee DA, Allgood D, Kneitel JM, Kuehn KA. 2012. “Constitutive differences between natural and artificial container mosquito habitats: vector communities, resources, microorganisms, and habitat parameters.” Journal of Medical Entomology 49:482–491. https://doi.org/10.1603/ME11227