The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids, and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Neohydronomus affinis Hustache is known as the waterlettuce weevil (suggested common name) (Figure 1). It is a biological control agent of the floating plant, waterlettuce, Pistia stratiotes Linnaeus (Araceae) (O’Brien and Wibmer 1989, Center et al. 2002). Waterlettuce is found in tropical and subtropical regions of the world. It can be found in slow-moving streams, waterways, lakes, and rivers, forming dense floating mats that are composed of several rosettes connected by stolons (runner stems). The presence of waterlettuce mats diminishes biological diversity and generates obstructions for boating, waterflow, and aquatic organisms (Cordo and Sosa 2000, Dray and Center 2002). Waterlettuce was documented by explorer William Bartram in Florida before 1765 (plants-archive.ifas.ufl/plant-directory). It is listed as a class II prohibited aquatic plant in the State of Florida, which means it is highly invasive and noxious in particular areas of Florida.
There are three species in the genus Neohydronmus, all of which originate from South and Central America. These weevils are subaquatic, and their exoskeletons are covered in white scales with no water-resistant features. The three species are all associated with waterlettuce and feed exclusively on it (Center et al. 2002, O’Brien and Wibmer 1989). Among them, Neohydronomus affinis was first introduced as a biological control agent into Florida in 1987 at Torry Island and Kreamer Island at the south end of Lake Okeechobee (Dray et al. 1990, 2001). Since then, the insect has successfully established itself in peninsular Florida and is also reported in Louisiana (Center et al. 2002).

Credit: USDA ARS, USDA Agricultural Research Service, Bugwood.org, used with permission
Distribution
The waterlettuce weevil is native to South and Central America. This weevil was studied as a promising biological control candidate in 1976 (DeLoach et al. 1976). It has been successfully established in Australia (1984), Papua New Guinea, South Africa (1987), and the United States, and has helped suppress populations of waterlettuce (Figure 2) (Dray et al. 1990, Harley et al. 1990). In the United States, the insect has been effectively dispersed in Florida and Louisiana (Center et al. 2002).
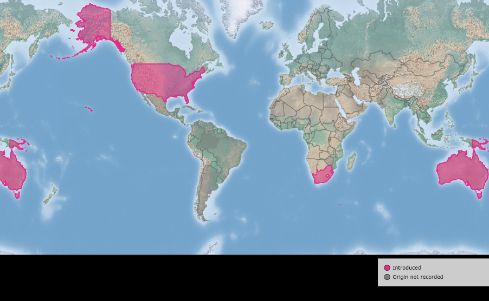
Credit: Center for Agriculture and Bioscience International (CABI). (As of January 10, 2020, https://www.cabi.org/isc/datasheet/36285#REF-DDB-151459)
Description
Adults
The adults of Neohydronomus affinis are small, ranging from 1.8 to 3 mm in length. Females are commonly larger than males, and the female to male ratio is one:one in the field. Adults vary from blue-grey to red-brown in color (DeLoach et al. 1976). Their dorsal surface is covered with erratic dense white scales without a waterproof coating, creating a chevron-like band pattern on the elytra, which are the sclerotized (hardened) protective forewing cases of a beetle. The appearance of the pattern on the dorsal thorax and elytra is not always easy to distinguish because of its being muddy or faded. The rostrum (a snout-like mouthpart) of Neohydronomus affinis is almost straight and long (~0.83 mm), which becomes narrow ventrally at the base (DeLoach et al. 1976, O’Brien and Wibmer 1989, Dray and Center 2002).
Eggs
The egg is creamy in color and not quite spherical in shape, and measures around 0.33 mm × 0.44 mm. The egg is laid in the puncture of the upper surface of a young waterlettuce leaf, which is chewed by the female (Center et al. 2002, DeLoach et al. 1976).
Larvae
The larvae have creamy white to yellow bodies and orange to black heads as they develop (Figure 3) (Spinner 2022). There are three instars, all of which develop beneath the epidermis of the waterlettuce leaves. The newly-hatched (first instar) larvae initially have very small heads (0.2 mm in diameter). Larvae with head capsules 0.25 to 0.27 mm in diameter are considered second instars, and individuals with a range of 0.32 to 0.37 mm are third instars, which reach a maximum length of 2.5 to 3 mm (Dray and Center 2002).

Credit: USDA ARS, USDA Agricultural Research Service, Bugwood.org, used with permission
Pupae
The pupae are creamy white, are wider than the larvae and display black spots on their heads (Figure 3). The naked pupae, which are pupae without a cocoon or outer covering, complete their development in the internal spongelike tissues of the waterlettuce leaf (Dray and Center 2002).
Life Cycle
The life cycle of Neohydronomus affinis is separated into four unique stages, including egg, larva, pupa, and adult.Before oviposition, adult females of the waterlettuceweevilsminecircularholesabout 0.5 mm in diameterbychewing the leaf surface (DeLoach et al. 1976, Dray and Center 2002). These circular holes in the thin layer of the leaf edge maypenetratethroughout the whole leaf, otherwise the holes areshallow.The female deposits only one egginside of the shallow hole, near the margin of the upper leaf of the young waterlettuce,and subsequently coversit with frass(Moore and Hill 2012, Harley et al. 1990).
Optimum temperatures for hatching eggs are above 24°C, and within four days, the newly hatched larvae start boring into the leaf. The larvae molt three times, where they excavate and feed on the spongelike tissues inside the leaf at a rate of 15 to 20 mm per day. Larval development continues in the leaf for 11 to 14 days as the feeding moves toward the spongy tissues. Consequently, pupation occurs within the larval mine in the leaf. Adults emerge after four to five days, and the newly emerged adults move to the surface of the leaf to feed (Harley et al. 1990).
The adults of Neohydronomus affinis begin to mate, oviposit, and produce offspring, and then the adults progressively chew and burrow within the internal spongy tissues of the leaf. Their whole life cycle, from egg to adult, occurs over the course of 16 to 24 days. The shortest period of completing the life cycle for Neohydronomus affinis appears during summer, while slow development of the weevils is seen during cooler seasons (spring, fall, and winter) (Cilliers 1987, DeLoach et al. 1976, Harley et al. 1990).
Host
The origin of waterlettuce is not known (Adebayo et al. 2011), but the plant occurs worldwide (Figure 4). Waterlettuce weevils mainly feed on waterlettuce, which is considered one of the world’s worst weeds (Dray and Center 2002, Holm et al. 1977). Besides waterlettuce, adult weevils feed slightly on other plants such as Spirodela (duckweed), Lemna (duckweed), and Limnobium (American frogbit or spongeplant) that are in association with the habitat of the waterlettuce. However, females of Neohydronomus affinis do not lay eggs on any plant species, except waterlettuce (DeLoach et al. 1976).
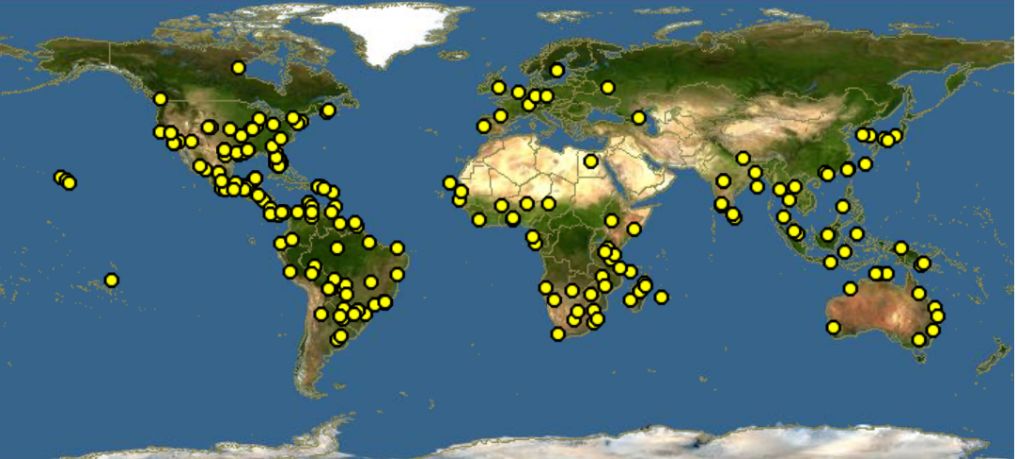
Credit: Discover Life (Last updated 25 February 2022 at: https://www.discoverlife.org/mp/20m?kind=Pistia+stratiotes)
Damage
The adults of the waterlettuce weevil damage both the external and internal portions of the leaves, creating 1.4 mm feeding holes, but the larvae feed only inside the leaves, producing tunnels (Figure 5). On the thin tissues of leaves, larval tunnels can be seen, but they are not visible in the middle and bottom of the leaves (Center et al. 2002, Harley et al. 1990). Adults and larvae impede the growth of leaves on the plant, resulting in small leaves and a reduction in the number of leaves. As a result, compared to unaffected plants, the floating mat densities fall, and the damaged water lettuce populations expand more slowly. The presence of feeding holes on the epidermal surface of leaves and tunnels inside the spongy tissue of leaves provide suitable conditions for introducing water into the leaves. Consequently, plants may no longer be buoyant and sink to the bottom of the water body. In some locations where insect populations are high, larvae and adults produce severe damage and cause plant diebacks, whereas low populations are hard to detect (Center et al. 2002, DeLoach et al. 1976). Waterlettuce root damage through feeding of waterlettuce weevils has not been reported.

Credit: USDA ARS, USDA Agricultural Research Service, Bugwood.org, used with permission
Importance as a Biological Control Agent
In various countries, including Australia, Benin, Botswana, Cote d’lvoire, Senegal, Zimbabwe, Zambia, and the United States of America, Neohydronomus affinis is a valuable biological agent used to control waterlettuce (Winston et al. 2014). This weevil diminished waterlettuce infestations by 40% or more in Australia between 12 and 18 months after release (Harley et al. 1990). During the first year of application of the biological control agent, waterlettuce weevils nearly eradicated Pistia stratiotes from a heavily affected area in South Africa (Cilliers 1987). The waterlettuce weevil is an effective biological control agent in Florida, causing up to 90% decline in waterlettuce populations at five different locations (south central) in Florida and two regions in Louisiana (Dray and Center 1992). Weevil populations fluctuate in tropical areas due to seasonal conditions, particularly cooler winters, and the weevils can effectively control waterlettuce after 18-24 months of establishment (Cilliers and Mabulu 2002, Harley et al. 1990). Although waterlettuce propagates easily through stolons or viable seeds in the soil, the release of the waterlettuce weevil aids in the successful control of this aquatic plant.
Selected References
Adebayo AA, Briski E, Kalaci O, Hernandez M, Ghabooli S, Beric B, Chan F, Zhan A, Fifield E. 2011. Water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes) in the Great Lakes: Playing with fire? Aquatic Invasions, 6(1), 91. https://doi.org/10.3391/ai.2011.6.1.11
Center T, Dray F, Jubinsky G, Grodowitz M. 2002. Insects and other arthropods that feed on aquatic and wetland plants. United States Department of Agriculture. Agricultural Research Service, Technical Bulletin.
Cilliers CJ. 1987. First attempt at and early results on the biological control of Pistia stratiotes L. in South Africa. Koedoe, 30(1), 35-40. https://doi.org/10.4102/koedoe.v30i1.500
Cordo HA, Sosa A. 2000. The Weevils Argentinorhynchus breyeri, A. bruchi and A. squamosus (Coleoptera: Curculionidae), Candidates for the Biological Control of Waterlettuce (Pistia stratiotes). In Proceedings of the X International Conference on Biological Control of Weeds, 4-14.
DeLoach CJ, DeLoach AD, Cordo HA. 1976. Neohydronomus pulchellus 1, a Weevil Attacking Pistia stratiotes in South America: Biology and Host Specificity 2, 3. Annals of the Entomological Society of America, 69(5), 830-834. https://doi.org/10.1093/aesa/69.5.830
Dray FA, Center T. 2002. Water lettuce. Biological Control of Invasive Plants in the Eastern United States; Driesche, RV; Blossey, B, 65-78.
Dray FA, Center TD. 1992. Biological control of Pistia stratiotes L. (waterlettuce) using Neohydronomus affinis Hustache (Coleoptera: Curculionidae). Aquatic Weed Research Laboratory US Department of Agriculture, Agriculture Research Service Fort Lauderdale, Florida.
Dray FA, Center, TD, Habeck DH, Thompson CR, Cofrancesco AF, Balciunas JK. 1990. Release and Establishment in the Southeastern United States of Neohydronomus affinis (Coleoptera: Curculionidae), an Herbivore of Waterlettuce. Environmental Entomology, 19(3), 799-802. https://doi.org/10.1093/ee/19.3.799
Dray FA, Center TD, Wheeler GS. 2001. Lessons from Unsuccessful Attempts to Establish Spodoptera pectinicornis (Lepidoptera: Noctuidae), a Biological Control Agent of Waterlettuce. Biocontrol Science and Technology, 11(3), 301-316. https://doi.org/10.1080/09583150120055718
Harley K, Forno I, Kassulke R, Sands D. 1984. Biological control of water lettuce. Journal of Aquatic Plant Management 22: 101-102.
Harley K, Kassulke R, Sands D, Day M. 1990. Biological control of water lettuce, Pistia stratiotes [Araceae] by Neohydronomus affinis [Coleoptera: Curculionidae]. Entomophaga, 35(3), 363-374. https://doi.org/10.1007/BF02375260
Holm LG, Plucknett DL, Pancho JV, Herberger JP. 1977. The world's worst weeds. Distribution and biology. University press of Hawaii.
Moore GR, Hill MP. 2012. A Quantitative Post-Release Evaluation of Biological Control of Water Lettuce, Pistia Stratiotes L. (Araceae) by the Weevil Neohydronomus Affinis Hustache (Coleoptera: Curculionidae) at Cape Recife Nature Reserve, Eastern Cape Province, South Africa. African Entomology, 20(2), 380-385. https://doi.org/10.4001/003.020.0217
O'Brien CW, Wibmer GJ. 1989. Revision of the Neotropical Genus Neohydronomus Hustache (Coleoptera: Curculionidae). The Coleopterists Bulletin, 43(3), 291-304. https://doi.org/10.1093/aesa/82.3.267
Winston RL, Schwarzländer M, Hinz HL, Day MD, Cock MJ, Julien MH. 2014. Biological control of weeds. A World Catalogue of Agents and Their Target Weeds (Fifth Edition). USDA Forest Service, Forest Health Technology Enterprise Team, Morgantown, West Virginia.
Spinner S. 2022. February 22. LSU AgCenter. Retrieved from lsuagcenter: https://www.lsuagcenter.com/topics/environment/invasive%20species/water-lettuce/biological-control.
UFIFAS. https://plants-archive.ifas.ufl.edu/plant-directory/pistia-stratiotes/