Introduction
Neomusotima conspurcatalis (Warren) (Lepidoptera: Crambidae) is an imported biological control agent of Old World Climbing Fern, Lygodium microphyllum (Cav.) R. Br. (Lygodiaceae) (Figure 1).

Credit: Ellen Lake, USDA-ARS
Adult moths were initially released in 2008 on a population of Lygodium microphyllum at Jonathan Dickinson State Park in Martin County (Boughton & Pemberton 2009). The released insects were originally collected from northern Queensland, Australia (Boughton & Pemberton 2009). As of 2014, populations of N. conspurcatalis were established at Jonathan Dickinson State Park (Martin County), Loxahatchee National Wildlife Refuge (Palm Beach County), Cypress Creek Preserve (Pasco County) and Flatford Swamp (Manatee County) (Smith et al. 2014).
Lygodium microphyllum is a non-native climbing fern that grows vertically through the tree canopy and spreads horizontally, facilitating its dominance in canopy and understory plant communities (Mueller 1982, Pemberton & Ferriter 1998, Lott et al. 2003) (Figure 2). Lygodium microphyllum spreads rhizomatously, i.e., through root sprouts, and via self-fertilizing spores produced by fertile leaflets, or pinnae (Mueller 1982). These spores can be carried into undisturbed natural areas where they can establish new populations of Old World Climbing Fern (Mueller 1982).

Credit: Ellen Lake, USDA-ARS
First identified near Jupiter, Florida in 1968, Lygodium microphyllum spread throughout moist habitats in central and southern Florida (Boughton et al. 2009). Since Lygodium microphyllum can regrow from rhizomes, neither mechanical removal nor burning are completely effective control strategies (Goolsby et al. 2003). Herbicides are effective at killing above-ground foliage, but costly re-applications are required to achieve longer term control due to the inability of herbicides to translocate to the rhizome (Hutchinson et al. 2006, Hutchinson et al. 2010). In Florida, Lygodium microphyllum covers 800,000 hectares in some of the most remote communities and habitats, from the Florida Keys to near the Georgia border (Figure 3), disrupting fire and hydrological cycles (Schmitz et al. 1997, Rodgers et al. 2014). Lygodium microphyllum requires an integrated sustainable long-term management strategy achieved through the addition of biological control (i.e., Boughton & Pemberton 2009).
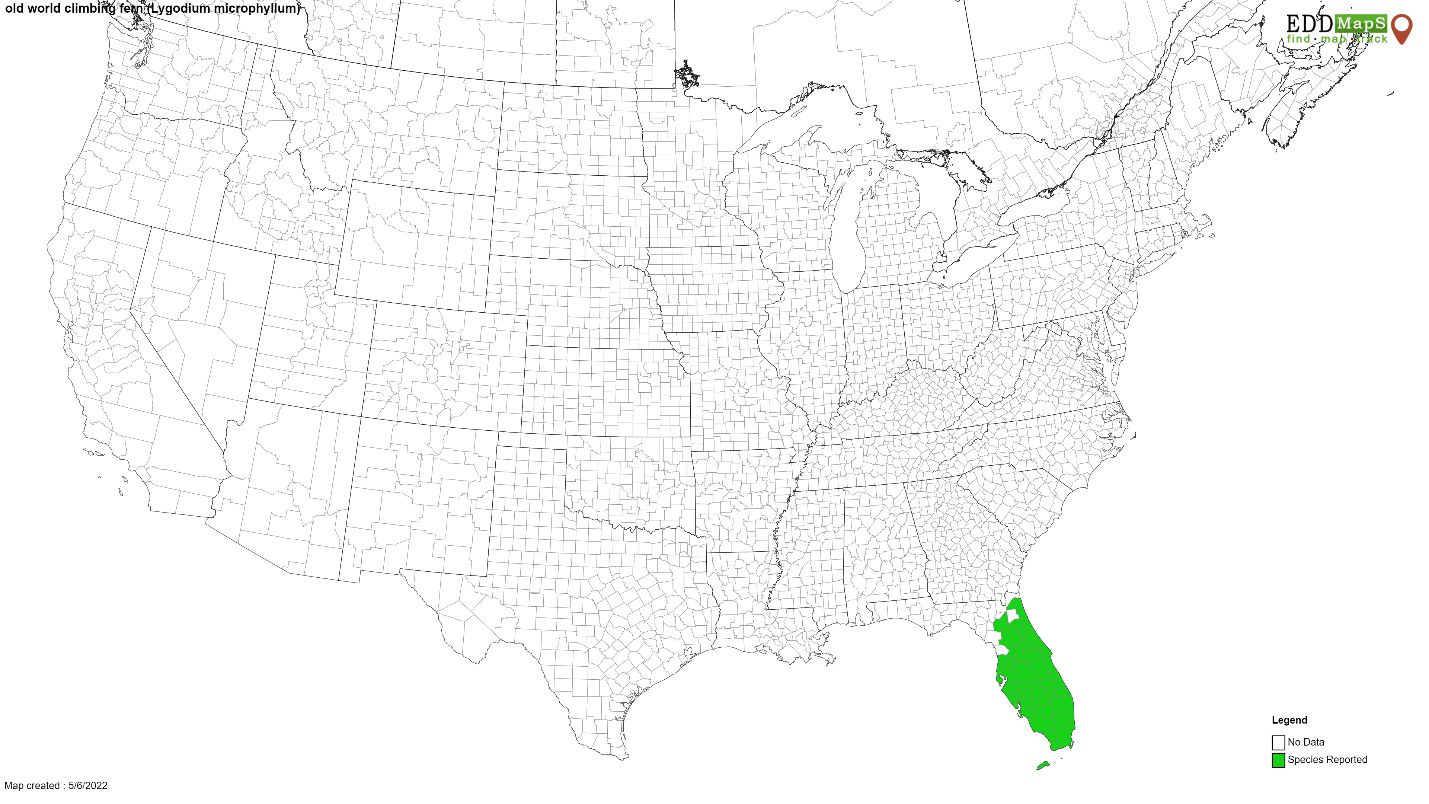
Credit: www.eddmaps.org
Distribution
Neomusotima conspurcatalis has a broad native distribution. Populations are found as far north as northeastern India and southern China, at a latitude of approximately 27°N, and as far south as Northern Territory and Western Australia at around 15°S (Solis et al. 2004, Boughton et al. 2009).
In its introduced range in the United States, Neomusotima conspurcatalis is restricted to Florida and evidence suggests that cold temperatures may limit its northward spread (Smith et al. 2014). In laboratory studies, development of Neomusotima conspurcatalis slows substantially at temperatures fall below 26.5°C (80°F), which may limit its distribution to central and southern Florida (Boughton & Pemberton 2012). Cold winter temperatures in southern Florida in 2010 and 2011 reduced Neomusotima conspurcatalis populations, but moths were present in all release sites again by 2012-2013, except for Everglades National Park and three additional sites that had received herbicide treatment (Smith et al. 2014).
To increase the population of Lygodium defoliator moths, a mass rearing and release program for the moth began in 2014 (Jones et al. 2021). Neomusotima conspurcatalis is established in southern and central Florida, but establishment success has been inconsistent, with moth populations failing to establish at some locations within this range (Smith et al. 2014). The populations of Neomusotima conspurcatalis continue to expand in south and central Florida, leading to more frequent outbreaks and damage to Lygodium microphyllum (Jones & Lake 2020).
Description
Eggs
The yellowish eggs are flattened and oval, averaging 0.75 mm (1/32 in) in length (Boughton & Pemberton 2012) (Figure 4).

Credit: Ellen Lake, USDA-ARS
Larvae
The first instars are about 2 mm long and yellowish in color, with a black head capsule. The first three instars feed on the underside of the leaf, leaving little ‘windows’ of epidermal cells on the top side of the leaf (Boughton & Pemberton 2009, 2012) (Figure 5). Fourth and fifth instars are bright green and can feed on the edges of leaves, consuming entire pinnulets or leaflets (Boughton & Pemberton 2012). Later instars may also skeletonize leaves under a disguise of frass and silken threads that the caterpillars create across the lower edges of the pinnulets (Figure 6) (Boughton & Pemberton 2012). At 26.5°C (80°F), most larvae pass through five instars in about 10 days (Boughton & Pemberton 2012).

Credit: Ellen Lake, USDA-ARS

Credit: Melissa Smith, USDA-ARS
Pupae
At the end of the fifth instar, the pre-pupae spin cocoons on the Lygodium stem or in leaf debris (Boughton & Pemberton 2012). The pupae are dark brown and 6-7 mm long (Boughton & Pemberton 2012). The pupal stage lasts about 5 days at 26.5°C (80°F) (Boughton & Pemberton 2012).
Adults
Adults of Neomusotima conspurcatalis are brown with a distinctive white ‘boomerang’ mark on the tip of each forewing (Fig. 1) (Boughton & Pemberton 2009, 2012). The wingspan is 11 mm (Boughton & Pemberton 2009, 2012).
Life Cycle and Biology
Neomusotima conspurcatalis adults emerge at night and mate once, on the first night after emergence (Boughton & Pemberton 2012). The next night, the female moth will lay about half of her lifetime supply of eggs (Boughton & Pemberton 2012). Mated female Neomusotima conspurcatalis lay 65-172 eggs in their lifetime (Boughton & Pemberton 2012). A female moth will deposit her eggs singly or in small, overlapping clusters on the leaves of Lygodium microphyllum (Boughton & Pemberton 2009, 2012). Female Neomusotima conspurcatalis prefer to oviposit on fertile, spore-producing leaflets that are less abundant than sterile leaflets (Smith et al. 2016). Caterpillars feed on the underside of Lygodium microphyllum leaflets, leaving behind the epidermal leaf tissue on the top side of the leaf (Boughton & Pemberton 2009) (Figure 5). Caterpillars often pupate in webbing and frass on the undersides of leaves. At 25°C (77°F), development of Neomusotima conspurcatalis takes about 30 days from egg to adult (Boughton & Pemberton 2009). Adults are short-lived, surviving about 10 days (Boughton & Pemberton 2009). Neomusotima conspurcatalis is active year-round in Florida, but flourishes during fall and spring shoulder seasons (Boughton & Pemberton 2009). Where populations have been successful, Neomusotima conspurcatalis can amass on Old World Climbing Fern and cause patches of Lygodium to turn brown, in what are termed “brown out” events (Boughton & Pemberton 2009) (Figure 7).

Credit: Aaron David, USDA-ARS
Hosts
The search for a host specific biological control agent for Lygodium microphyllum began in 1997 (Goolsby et al. 2003). Surveys were conducted in Australia, Hong Kong, Japan, Indonesia, Malaysia, New Caledonia, Singapore, Taiwan, Thailand, and Vietnam (Goolsby et al. 2003). During these surveys, two mite and 20 insect species were collected feeding on Lygodium microphyllum the related fern species, including Lygodium reticulatum Schkuhr, Lygodium japonicum (Thunb.), and L. flexuosum (L.) Sw. (Goolsby et al. 2003). In the field, Neomusotima conspurcatalis and its feeding damage were observed only on Lygodium microphyllum but not on nearby Lygodium species (i.e., Lygodium reticulatum, Lygodium flexuosum, or Lygodium japonicum) (Goolsby et al. 2003). Neomusotima conspurcatalis was frequently found during collecting surveys and was present throughout the range of Lygodium microphyllum in Southeast Asia and Australia, although not in subtropical Queensland and New South Wales (Goolsby et al. 2003). In preliminary laboratory host range testing, Neomusotima conspurcatalis laid eggs on 12 fern species, but larvae only developed to the adult stage on Lygodium microphyllum, Lygodium japonicum and Lygodium palmatum (Goolsby et al. 2003). Despite the ability to develop on several Lygodium species under lab conditions, Neomusotima conspurcatalis larvae and their distinctive damage have never been observed on Lygodium species other than Lygodium microphyllum during native range field surveys (Goolsby et al. 2003).
Potential weed biological control agents are identified in field surveys and undergo preliminary field host range testing. After preliminary testing, promising biological control agents undergo laboratory host range testing. Laboratory host range testing is designed to determine the range of plants on which the insects will feed and develop. In the case of Neomusotima conspurcatalis, 48 plant species were fed to groups of Neomusotima conspurcatalis as part of host range testing in a Florida quarantine facility (Boughton et al. 2009). Non-target species of Lygodium tested were the non-native Japanese climbing fern, Lygodium japonicum and Lygodium palmatum; the latter is the only Lygodium fern native to the United States. Lygodium palmatum is a temperate plant, and natural (non-cultivated) populations of the fern exist only as far south as northern Alabama, Georgia and South Carolina. Cold tolerance tests of Neomusotima conspurcatalis indicate that the moth would not survive in the northern part of Florida (north of Dayton or Tampa) (Boughton et al. 2009). The four other Lygodium species tested occur in the West Indies and Central and South America (Boughton et al. 2009).
Host range testing also included 37 species of non-Lygodium ferns that grow in Florida. The ferns that were tested include both native ferns, some of which are threatened or endangered, as well as ferns that are common horticultural plants (Boughton et al. 2009). Rice, citrus, and sugarcane plants were included in host range testing because they are agriculturally important in Florida. Bald cypress, a common and ecologically significant tree in habitats invaded by Lygodium microphyllum, was also tested (Boughton et al. 2009). Neomusotima conspurcatalis did not attack any of these plant species.
Economic Importance
Old World Climbing Fern is an expensive plant to manage. The state of Florida spends approximately $2 million annually to control Lygodium microphyllum on state conservation lands (Hiatt et al. 2019). Ongoing efforts to improve the control of Old World Climbing Fern include mass releasing of biological control agents as well as improvements to release strategies and integrating biological control with other weed management techniques.
The results of several experiments suggest that environmental conditions and additional plant stress can act together to improve control of Old World Climbing Fern by Neomusotima conspurcatalis. Old World Climbing Fern plants grown under full sun conditions suffered no mortality even after being fully defoliated by Neomusotima conspurcatalis. However, almost one-third of plants grown under shady conditions and subject to Neomusotima conspurcatalis herbivory died (Jones & Lake 2020).
Prescribed burning can kill unwanted vegetation and stress remaining plants, which may make them more susceptible to biological control agents. Neomusotima conspurcatalis recolonized burned patches of Old World Climbing Fern within five months of controlled burns, indicating that use of Neomusotima conspurcatalis is compatible with prescribed burns to control Old World Climbing Fern (David et al. 2020).
Lake at al. (2020) showed that drone releases have the potential to improve establishment of Neomusotima conspurcatalis in Florida. In a comparison of releases by drone and by hand, Lake et al. (2020) recovered more larvae of Neomusotima conspurcatalis dropped into infestations of Lygodium microphyllum via drone than when larvae were transferred by hand into infestations. This is important because Lygodium microphyllum grows in many conservation areas that are difficult to access and populated with dangerous wildlife (Lake et al. 2020).
Management
Within three years of introduction to Florida, Neomusotima conspurcatalis was attacked by larval parasitoids (Boughton et al. 2012). These parasitoids were the native hymenopteran parasitoids Elasmus apanteli Gahan (Eulophidae), Mesochorus apantelis Dasch (Ichneumonidae), Stantonia pallida (Ashmead), Rhygoplitis choreuti (Viereck), and Cotesia sp. of unknown origin (all Braconidae). The native fly Hyphantrophaga sellersi (Sabrosky) (Diptera: Tachinidae) also attacks Neomusotima conspurcatalis larvae (Boughton et al. 2012). Despite the high number of generalist parasitoid species that attack Neomusotima conspurcatalis, overall parasitism rates are low, suggesting that larval parasitism is not regulating populations of the weed biological control agent in Florida (Boughton et al. 2012).
Neomusotima conspurcatalis is also attacked by an egg parasitoid Trichogramma sp. (Hymenoptera: Trichogrammatidae), which, in some samples, parasitized close to 100% of the eggs in an egg mass (Lake et al. 2015). Additional research is required to determine how this egg parasitoid may affect the population dynamics of Neomusotima conspurcatalis (Lake et al. 2015).
Predation may be an important component factor in preventing the establishment of Neomusotima conspurcatalis in some locations. Larval Neomusotima conspurcatalis were consumed by ants, spiders, cockroaches and beetles in laboratory tests (Jones et al. 2021). In field plots where Neomusotima conspurcatalishad been released, ants and spiders were the most common predators, and had the highest predation rates (Jones et al. 2021).
Selected References
Boughton A, Bennett C, Goolsby J, Pemberton R, 2009. Laboratory host range testing of Neomusotima conspurcatalis (Lepidoptera: Crambidae) a potential biological control agent of the invasive weed, Old World climbing fern, Lygodium microphyllum (Lygodiaceae). Biocontrol Science and Technology 19, 369-390. https://doi.org/10.1080/09583150902771194
Boughton A, Kula R, Gates M, Zhang Y, Nunez M, 2012. Parasitoids attacking larvae of a recently introduced weed biological control agent, Neomusotima conspurcatalis (Lepidoptera: Crambidae); key to species, natural history, and integrative taxonomy. Annals of the Entomological Society of America 105, 753-767. https://doi.org/10.1603/AN11157
Boughton A, Pemberton R, 2009. Establishment of an imported natural enemy, Neomusotima conspurcatalis (Lepidoptera: Crambidae) against an invasive weed, Old World climbing fern, Lygodium microphyllum, in Florida. Biocontrol Science and Technology 19, 769-772. https://doi.org/10.1080/09583150903100823
Boughton A, Pemberton R, 2012. Biology and reproductive parameters of the brown Lygodium moth, Neomusotima conspurcatalis- a new biological control agent of Old World climbing fern in Florida. Ecological Entomology 41, 308-316. https://doi.org/10.1603/EN11146
David AS, Sebesta N, Abdel-Kader AA, Lake EC, 2020. Colonization by biological control agents on post-fire regrowth of invasive Lygodium microphyllum (Lygodiaceae). Environmental Entomology 49, 796-802. https://doi.org/10.1093/ee/nvaa076
EDDMapS. 2022. Early Detection & Distribution Mapping System. The University of Georgia - Center for Invasive Species and Ecosystem Health. Available online at http://www.eddmaps.org/; last accessed May 6, 2022.
Goolsby J, Wright A, Pemberton R, 2003. Exploratory surveys in Australia and Asia for natural enemies of Old World climbing fern, Lygodium microphyllum: Lygodiaceae. Biological Control 28, 33-46. https://doi.org/10.1016/S1049-9644(03)00054-9
Hiatt D, Serbesoff-King K, Lieurance D, Gordon DR, Flory SL, 2019. Allocation of invasive plant management expenditures for conservation: Lessons from Florida, USA. Conservation Science and Practice, e51. https://doi.org/10.1111/csp2.51
Hutchinson J, Ferriter A, Serbesoff-King K, Langeland K, & Rodger, L 2006. Old World climbing fern (Lygodium microphyllum) management Plan for Florida (2nd ed.). Florida Exotic Pest Plant Council.
Hutchinson J, Langeland KA, MacDonald GE, Querns R, 2010. Absorption and translocation of glyphosate, metsulfuron and triclopyr in Old World climbing fern (Lygodium microphyllum). Weed Science 58, 118-125. https://doi.org/10.1614/WS-D-09-00046.1
Jones IM, Lake EC, 2020. Defoliation of the invasive fern Lygodium microphyllum by Neomusotima conspurcatalis: Effects on plant performance across a range of light conditions. Biological Control 144, Article 104236. https://doi.org/10.1016/j.biocontrol.2020.104236
Jones IM, Madeira PT, Blair JZ, Lake EC, 2021. Using molecular gut content analysis to identify key predators in a classical weed biological control system: a study with Neomusotima conspurcatalis (Lepidoptera: Crambidae). BioControl 66, 825-836. https://doi.org/10.1007/s10526-021-10090-x
Lake E, David A, Spencer T, Wilhelm Jr, V, Barnett T, Abdel-Kader A, Cortes A, Acuna A, Mattison E, Minteer C, 2020. First drone releases of the biological control agent Neomusotima conspurcatalis on Old World climbing fern. Biocontrol Science and Technology. https://doi.org/10.1080/09583157.2020.1828280
Lake E, Gates M, Smith M, Witkus, G, Pratt P, 2015. First report of an egg parasitoid reared from Neomusotima conspurcatalis (Lepidoptera: Crambidae), a biological control agent of Lygodium microphyllum (Schizaeales: Lygodiaceae). Florida Entomologist 98, 1244-1246. https://doi.org/10.1653/024.098.0436
Lott M, Volin J, Pemberton R, Austin D, 2003. The reproductive biology of the invasive ferns Lygodium microphyllum and Lygodium japonicum (Schizaeaceae): implications for invasive potential. American Journal of Botany 90, 1144-1152. https://doi.org/10.3732/ajb.90.8.1144
Mueller R, 1982. Shoot morphology of the climbing fern Lygodium (Schizaeaceae): general organography, leaf initiation and branching. Botanical Gazette 143, 319-330. https://doi.org/10.1086/337306
Pemberton R, Ferriter A, 1998. Old World climbing fern (Lygodium microphyllum), a dangerous invasive weed in Florida. American Fern Journal 88, 165-175. https://doi.org/10.2307/1547769
Rodgers L, Pernas T, Hill S, 2014. Mapping invasive plant distributions in the Florida Everglades using the digital aerial sketch mapping technique. Invasive Plant Science and Management 7, 360-374. https://doi.org/10.1614/IPSM-D-12-00092.1
Schmitz D, Simberloff D, Hoffstetter R, Haller W, Sutton D, 1997. The ecological impact of non-indigenous plants [pp. 29-74]. In: Simberloff, D., Schmitz, D., Brown, T., Eds.), Strangers in Paradise. Island Press, Washington, D.C.
Smith M, Lake E, Pratt P, Boughton A, Pemberton, R., 2014. Current status of the biological control agent Neomusotima conspurcatalis (Lepidoptera: Crambidae), on Lygodium microphyllum (Polypodiales: Lygodiaceae) in Florida. Florida Entomologist 97, 817-820. https://doi.org/10.1653/024.097.0268
Smith MC, Lake EC, Wheeler GS, 2016. Oviposition choice and larval performance of Neomusotima conspurcatalis on leaflet types of the invasive fern, Lygodium microphyllum. Entomologia Experimentalis et Applica 160, 11-17. https://doi.org/10.1111/eea.12450
Solis M, Yen S-H, Goolsby J, 2004. Species of Lygomusotima new genus and Neomusotima Yoshiyasu (Lepidoptera: Crambidae) from Australia and Southeastern Asia feeding on Lygodium microphyllum (Schizaeaceae). Annals of the Entomological Society of America 97, 64-76. https://doi.org/10.1603/0013-8746(2004)097[0064:SOLNGA]2.0.CO;2