Abstract
Aedes pertinax is a mosquito species first described in Jamaica by Grabham in 1906. It is currently classified in the Protoculex Group. Aedes pertinax, and Aedes nubilus were considered synonyms with Aedes serratus until 1970 when Belkin et al. restored them to species status. This non-invasive species was first identified in the United States in 2015 from adults and larvae previously collected in Florida in 2011 (Shroyer et al. 2015). Since then, it has also been found in Collier County, Lee County, and Miami-Dade County, all in Florida (Riles and Conelly 2021; Tyler-Julian et al. 2022; Sloyer et al. 2022). The current distribution of Aedes pertinax in the United States is unknown due to the lack of identification resources that give identifiers the ability to separate it from other mosquito species that are morphologically similar. Little information is available about Aedes pertinax. It has not been reported in association with humans or animal diseases, but Aedes Atlanticus, another mosquito species in the Protoculex Group, is a vector of the Keystone virus (Henry et al. 2022) and the West Nile virus (CDC), which have the potential to cause encephalitis and death. Moreover, Aedes serratus, once classified as synonym of Ae. pertinax, was reported infected with yellow fever in Brazil in 2008 (Cardoso et al. 2010). Considering this, and the fact that Ae. pertinax has expanded its geographical range, it is important to closely monitor it, this requires the ability to Identify this species, which is the main focus of this paper.
Intended Audience and Purpose
This is an Aedes pertinax fact sheet for mosquito control professionals, students, scientists, and the general public This publication is structured similarly to those from Walter Reed Biosystematics Unit (WRBU) so that readers may obtain details similar to those offered through WRBU species pages (https://wrbu.si.edu/vectorspecies), which is a worldwide renowned platform dedicated to conducting laboratory and field research on the systematics of medically important arthropod species and widely used by anyone interested in mosquito biology, research, and education. Here we provide information about taxonomy, bionomics and distribution of Aedes pertinax adults and larvae that will help to identify this mosquito species and separate it from morphologically similar species.
Neartic and Neotropical Regions
Family: Culicidae
Subfamily: Culicinae
Tribe: Aedini
Genus: Aedes
Subgenus: Ochlerotatus
Group: Protoculex (Reinert et al. 2000)
Etymology
From Latin per- ("very") + tenāx (“tenacious”)
Brief Species Description
Aedes pertinax was originally identified in Jamaica by Grabham in 1906. It has also been found in other Caribbean countries including Haiti, Puerto Rico, the Dominican Republic, and the Bahamas, as well as the continental United States (Florida).
Type Locality
Kingston Jamaica
Type Depository
The Smithsonian Institution, formerly the United States National Museum, Washington D.C., United States (officially cited as USNM, reflecting its original name.)
Diagnostic Characters
Aedes pertinax (Grabham 1906) is a medium-sized mosquito with brown coloration due to the presence of a narrow, mid-longitudinal stripe of yellowish to white scales on the scutum (Figure 1). Aedes pertinax adults are very similar morphologically to Aedes atlanticus (Dyar and Knab 1906), Aedes dupreei (Coquillett 1904), and Aedes tormentor (Dyar and Knab 1906) in the Nearctic region, and Aedes aenigmaticus (Cerqueira and Costa 1946), Aedes eucephalaeus (Dyar 1919), Aedes hastatus (Dyar 1922), Aedes oligopisthus (Dyar 1918), Aedes serratus (Theoblad 1901), and Aedes nubilus (Theoblad 1903) in the West Indies and Neotropics (Belkin et al. 1970). In some instances, Aedes infirmatus, Aedes scapularis, and Aedes condolescens mosquitoes can resemble other species in the Protoculex Group (including Ae. pertinax), if specimens are damaged or weathered. However, distinctions between these similar-looking mosquito species can be made based on coloration of head scales; coloration; length and thickness of longitudinal stripe (scutum); presence of bands in the abdomen; and banding and claws in the legs. Adult female morphological characteristics used to differentiate these mosquitoes are summarized in Table 1.
Table 1. Adult female morphological characteristics of Aedes pertinax and other morphologically similar mosquitoes (Dyar 1921; Bonne and Wepster 1925; Belkin 1970; Arnell 1976; Consoli and Lourenco de Oliveira 1998; Darsie and Ward 2021; Wilkerson et al. 2021; Reeves et al. 2021).
Adult Females
Head
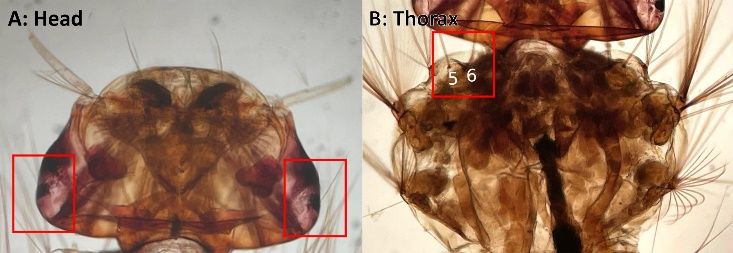
A broad patch of white to greyish scales is present on the head just before the prescutellar area, extending into the interocular space, and bordering the eyes. Whitish or with slight yellowish cast (Figure 1). The antenna is dark brown and filiform with silvery hairs on the joint.
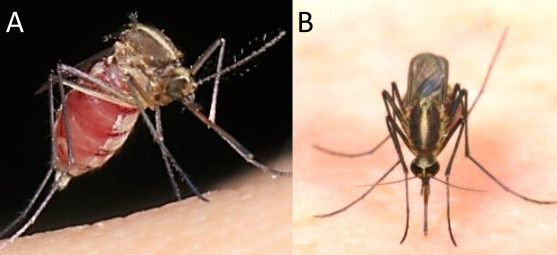
Credit: K. Sloyer, UF/IFAS, FMEL
Palpus
Three-segmented and entirely dark scaled; shorter than the proboscis.
Proboscis
Entirely dark scaled, rather short, subcylindrical, uniform. The labellae are conically tapered; the setae are minute and curved.
Thorax
The mesonotal scales are narrow, predominantly dark bronzy; sometimes a narrow acrostichal line of whitish scales is present. The lateral thoracic pleura has greyish to whitish scales and will never have silver scales. The scutum is entirely dark scaled with a narrow gray to white acrostichal line, which could have variations of orientation anteriorly (Figure 2). Dark bronzy scales are always present on the posterior doroscentral and prescutellar lines.
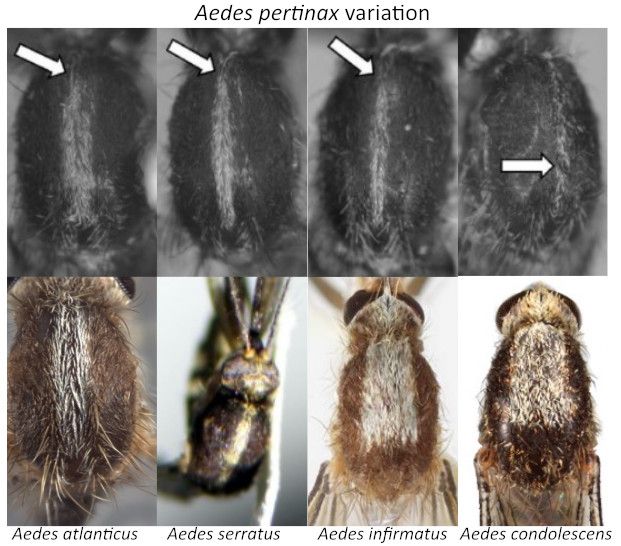
Credit: Top Row: modification from Shroyer et al. 2015, reproduced with permission from the American Mosquito Control Association; Bottom Row: Aedes atlanticus modified from Walter Reed Biosystematics Unit [2023]. Aedes atlanticus species page. Walter Reed Biosystematics Unit website, https://www.wrbu.si.edu/vectorspecies/mosquitoes/atlanticus accessed March 2023; Aedes serratus BOLD Systems2021; Aedes infirmatus and Aedes condolescens: Nathan Burkett-Cadena, UF/IFAS, FMEL
Legs
The coxae have white to yellowish scales. Dark tibiae and tarsi claws with prominent subbasal tooth. Leg characteristics are not needed for differentiation from other Aedes species.
Abdomen
The abdominal tergites are mostly dark scaled with large basolateral patches of white to yellowish scales. The abdominal sternites are pale scaled with prominent apicolateral dark scales.
Wings
Approximately 4.0 mm long and dark scaled with a blue reflection along the costa and a few scattered pale scales observed at base of costa (Figure 3). Wing characteristics are not needed for differentiation from other Aedes species.
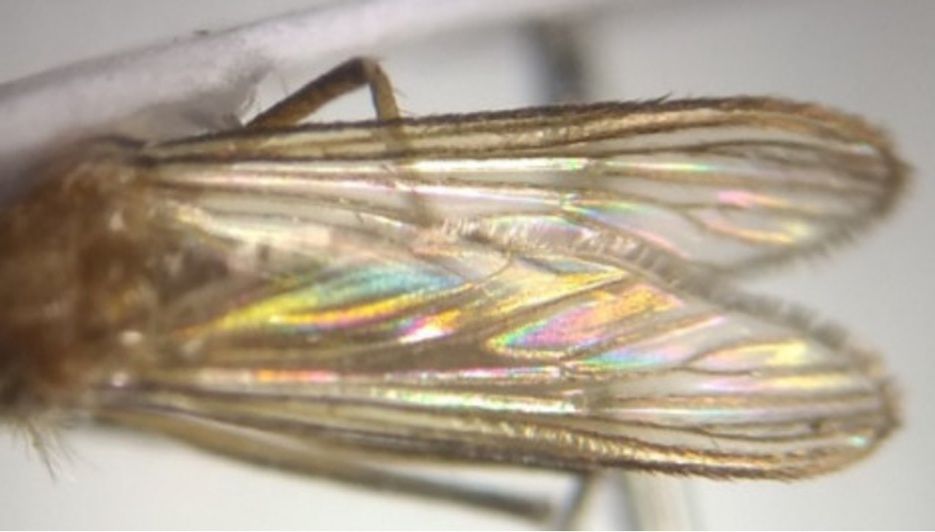
Credit: Boldsystems https://v3.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=1073464 License: CreativeCommons Attribution Non-Commercial Share-Alike (2019) https://creativecommons.org/licenses/by-nc-sa/3.0/legalcode License Holder: Unspecified, Laboratorio de Ecología de Enfermedades y Una Salud
Adult Males
Head
Scaling head predominantly pale except for erect scales. The antenna is plumose; the last two joints are black, slender rugose, and pilose; the others are pale, narrowly black ringed at insertions of hair-whorls; the hairs are long, black, and dense.
Palpus
Exceeds the proboscis by the length of the last joint. The apical part of the long joint and the last two joints are considerably swollen and with dense long black hairs.
Proboscis
Longer and slenderer than in females. Straight with bronzy-black scales.
Thorax
A band of conspicuous golden scales is present in the center.
Legs
The ungues of the fore and mid legs are unequal; the larger claw has two teeth and the smaller has one. The ungues of the hind legs are equal and unserrated.
Abdomen
Elongated and depressed, the lateral spots tending to form white basal segmental bands, abundant lateral ciliation, long, coarse, and pale. An acrostichal light-scaled line is present and apparently more frequently developed than in female.
Wings
The male wings are narrower than in the female, approximately 3.5 mm. The stalks of the fork-cell are longer. The vestiture is less abundant than in females.
Male Genitalia
The gonocoxite is elongated, three times as long as wide, and with numerous scales laterally and ventrally and occasionally with a few scales dorsally. The gonostylus is long and slender with a long articulate filament. The tergite lobe of segment IX is small but prominent. The thumblike apical tergomesal lobe is conspicuous with several short, thin setae. The sternomesal margin of the apical lobe has 2 irregular rows of bristles. The basal tergomesal lobe is detached (separated by a membrane) from the tergomesal margin of the side piece and joined to the base of the claspette tergally, with a small lobe bearing a long, specialized seta and 1 or 2 short, simple setae, sternally with a long, slender lobe bearing about 12 setae of varied lengths. The claspettes are slender and long but shorter than the gonostylus and form a roughly triangular shape, with a broad, sickle-shaped filament at the base. The aedeagus is moderately tapered. The proctiger is moderately developed (Figure 4).
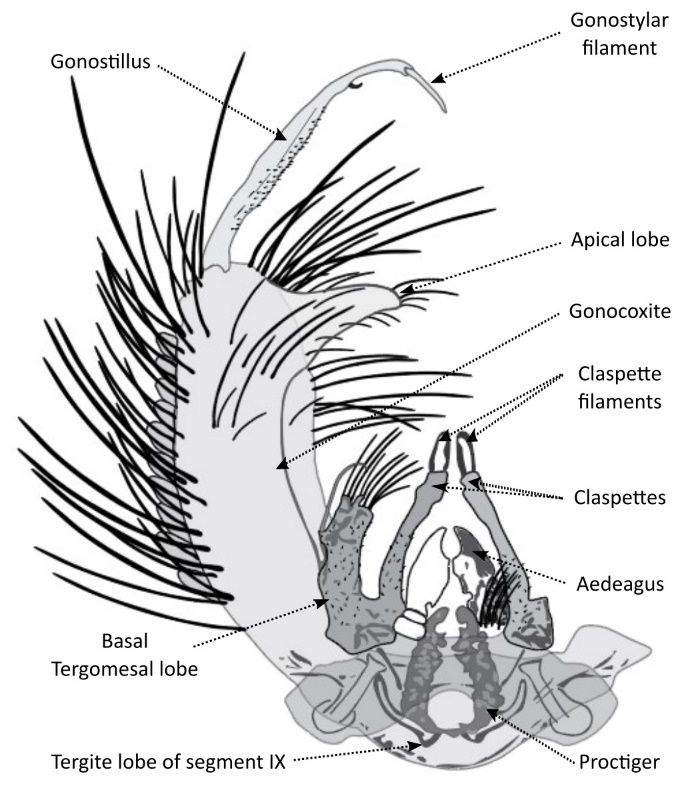
Credit: Daniel Killingsworth, Environmental Security Pest and Lawn, Cantonment, Florida
Larva
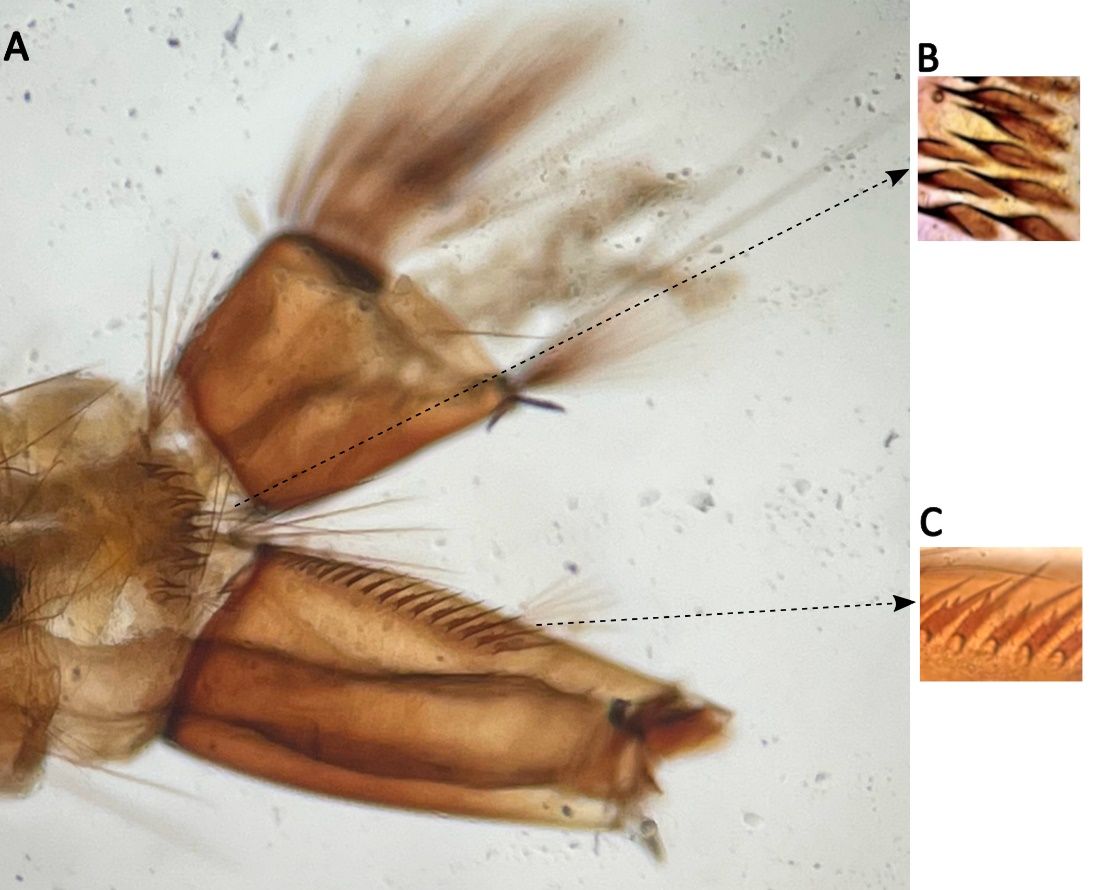
The Serratus Group species that include Aedes pertinax are characterized by a single row of spiney comb scales. The length of seta 6-C of dorsal apotome is 0.21–0.38; the bases of setae 5 and 6-P are connected by a narrow arcuate anterior plate; the comb is comprised of a single row of spine-like scales.
Credit: Mike Riles, Beach Mosquito Control District, Panama City, Florida
Head
Rounded, widest through eyes. The antenna is moderate, slender, and uniform with a small turf near the middle. Both pairs of dorsal head-hairs are single; the ante-antennal turf is multiple. Seta 4-C is conspicuously long. Denticles are present in the anterior ocular area (Figure 5A).
Thorax
Prothoracic setae 5 and 6-P are connected by a narrow, arcuate anterior plate (Figure 5B).
Abdomen
Lateral comb of eight abdominal segments with about 10 to 12 scales in a single irregular row, each scale with a long central spine. The sides are finely fringed.
Anal Segment and Siphon
Comb scales arranged in a single, irregular row (Segment VIII, Figure 6). Comb scales are large and fringed at base. The saddle and siphon are both dark and short, each with a black basal ring. Saddle encircles Segment X. Anal brush (4-X) with approximately eight pairs of branched setae on a grid. Two caudal setae (1,3-X) originating near dorsal brush (2-X) or piercing saddle (Figure 6). Uneven and long gills.
Credit: Mike Riles, Beach Mosquito Control District, Panama City, Florida
Pecten
With about ten evenly spaced teeth, ending before the middle of the siphon, it does not extend as far as that of Aedes tormentor. The tuft (1-S) is positioned at the last tooth.
Taxonomic Keys
Dyar 1918
Dyar 1922
Bonne & Wepster 1925
Belkin et al. 1970
Darsie et al. 2010
Howard et al. 1917
Exemplar DNA Sequences
Taxonomy ID 1640878
Gene Bank:
KP262407.1 — Ochlerotatus pertinax voucher IRCFL7 5.8S ribosomal RNA gene and internal transcribed spacer 2, partial sequence
KP262406.1 — Ochlerotatus pertinax voucher IRCFL6 5.8S ribosomal RNA gene and internal transcribed spacer 2, partial sequence
KP262405.1 — Ochlerotatus pertinax voucher JA792-3 5.8S ribosomal RNA gene and internal transcribed spacer 2, partial sequence
KP262404.1 — Ochlerotatus pertinax
voucher JA792-2 5.8S ribosomal RNA gene and internal transcribed spacer 2, partial sequence
Source: Shroyer et al. 2010
Bionomics
Little is known about the preferred larval and adult habitat, host-seeking behaviors, feeding preference, or seasonal distribution of Aedes pertinax.
Immatures
Belkin et al. (1970) reported a single collection of Aedes pertinax larvae in Jamaica from a coral rock hole filed with brackish water. Rueda Alarcon-Elba (2020) reported Aedes pertinax larvae found in temporary ground pools usually fully exposed to the sun. Shroyer et al. (2015) collected larvae from a cypress-oak-palm depression swamp in Indian River County, Florida.
Adults
Female adults were collected landing on humans in Jamaica (Belkin 1970). Adult specimens were collected using CDC miniature light traps baited with octenol on Lee Stocking Island and Gordo Cay, Bahamas (Darsie et al. 2010). Adult specimens were collected by aspiration from ground litter in a cypress-oak-palm depression swamp in Indian River County, Florida, and by CDC light trap, passive box, exit traps, and human landing catch collections (Shroyer et al. 2015). Giordano et al. (2021) collected a single specimen using a suction trap baited with 2 kg of dry ice in hydric hammock habitat in Indian River County. In Miami Dade County, adult mosquitoes were collected from tree buttresses, tree holes, animal burrows, and stream banks using a custom-made, small-diameter aspirator (Sloyer et al. 2022).
Associated Pathogens
None
Distribution Notes
Darsie et al. (2010); Belkin and Heinemann (1972); Belkin and Heinemann (1975); Belkin et al. (1970); Knight and Stone (1977); Shroyer et al. (2015); Rueda and Alarcón-Elbal (2020)
Distribution (Figure 7): Jamaica, Bahamas, Costa Rica, Cuba, Dominican Republic, Haiti, Puerto Rico, United Sates (Florida)
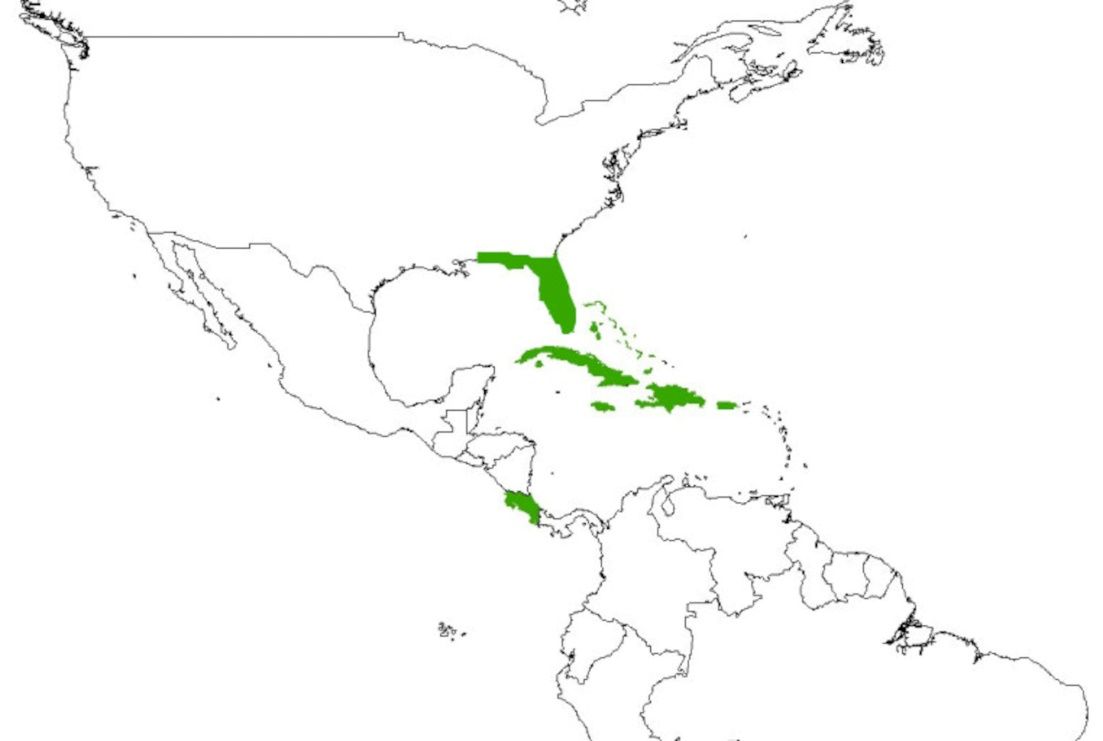
Credit: Created in ArcMap v. 10.7.1 using the World Countries (Generalized) product available directly from ESRI (https://hub.arcgis.com/datasets/esri::world-countries-generalized/about)
Available GIS Models
None as of December 2021.
Synonyms
The following were considered synonyms of Ae pertinax until 1970, when they were all restored to species by Belkin et al. (Belkin et al. 1970)
Aedes (Ochlerotatus) serratus (original combination: Culex serratus, Theobald, 1901)
Aedes (Ochlerotatus) (original combination: Culex nubilus, Theobald, 1903)
Protoculex (Ochlerotatus) pertinax (original combination: Protoculex quasiserratus, Theobald 1907)
Funding
We acknowledge funding support of the Southern IPM Center (Project S21-002) as part of USDA National Institute of Food and Agriculture Crop Protection and Pest Management Regional Coordination Program (Agreement No. 2018-70006-28884) and the USDA National Institute of Food and Agriculture (Hatch Multistate project 1025565).
Cited References
Arnell JH. 1976. Mosquito Studies (Diptera: Culicidae) XXXIII. A revision of the Scapularis group of Aedes (Ochlerotatus). Contributions of the American Entomological Institute, 3:1–144.
Belkin JN, Heinemann SJ, and Page WA. 1970. The Culicidae of Jamaica (Mosquito Studies. XXI). Contributions of the American Entomological Institute, 6:1–319.
Belkin J, Heinemann SJ, and Page WA. 1972. A Tentative Annotated List of the Culicidae of the Island of Hispaniola. Mosquito Systematics, 4: 63–72.
Belkin JN, Heinemann SJ, and Page WA 1975. Collection Records of the Project “Mosquitoes of Middle America” 3. Bahama Is. (BAH), Cayman Is. (CAY), Cuba (CUB), Haiti (HAC, HAR, HAT) and Lesser Antilles (LAR). Mosquito Systematics, 7:367–393.
Bonne C, and Bonne-Wepster J. 1925. Mosquitoes of Surinam. A study of neotropical mosquitoes. Royal Colonial Institute of Amsterdam. Druk de Bussy, Amsterdam, Netherlands, P. 397–409. Available online: https://books.google.com/books/about/Mosquitoes_of_Surinam.html?id=qa0LAQAAIAAJ (Accessed on 26 July 2022).
Cardoso JdaC, de Almeida MA, dos Santos E, da Fonseca DF, Sallum MA, Noll CA, Monteiro HA, Cruz AC, Carvalho VL, Pinto EV, Castro FC, Nunes Neto JP, Segura MN, and Vasconcelos PF. 2010. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, southern Brazil, 2008. Emerging Infectious Diseases.16:1918–24. https://doi.org/10.3201/eid1612.100608
Cerqueira NL and Costa AF. 1946 Duas novas especies de Aedes (Ochlerotatus) (Diptera Culicidae). Livro de homenagem a RF d'Almeida 11:133–140, illus. Available online: https://arim.ird.fr/arim/ (Accessed on 26 July 2022).
CDC website. Mosquito species in which West Nile virus has been detected, United States, 1999–2016 https://www.cdc.gov/westnile/resources/pdfs/MosquitoSpecies1999-2016.pdf (accessed on 4 Ausgust 2022).
Consoli RAGB and Lourenco de Oliveira R. 1998. Principais Mosquitos de Importancia Sanitaria No Brazil. Fundacao Oswaldo Cruz. Rio de Janeiro, Brazil, P. 108.
Coquillett DW. 1904. Several new Diptera from North America. Canadian Entomologist 1:10–12. https://doi.org/10.4039/Ent3610-1
Darsie RF Jr, Taylor S, Prusak ZA, and Verna TN. 2010. Checklist of the mosquitoes of the Bahamas with three additions to its fauna and keys to the adult females and fourth instars. Journal of the American Mosquito Control Association, 26:127–134. https://doi.org/10.2987/09-5982.1
Dyar HG. 1918. The male genitalia of Aedes as indicative of natural affinities. Insectuor Insectitiae Menstruus 6:71–86. https://doi.org/10.5962/bhl.part.14936
Dyar HG. 1919. New American Mosquitoes. Insectuor Insectitiae Menstruus. 6:120–129. https://doi.org/10.5962/bhl.part.14938
Dyar HG. 1922. The American Aedes of the Serratus Group. InsecutorInscitiaeMenstruus 10:157–166. Available online: https://mosquito-taxonomic-inventory.myspecies.info/node/11459 (Accessed on 26 July 2022).
Dyar HG and Knab F. 1906. The larvae of Culicidae classified as independent organisms. Journal of the New York Entomological Society 14:169–230. Available online: https://mosquito-taxonomic-inventory.myspecies.info/larvae-culicidae-classified-independent-organisms (Accessed on 26 July 2022).
Giordano BV, Cruz A, Pérez-Ramos DW, Ramos MM, Tavares Y, Caragata EP. Mosquito Communities Vary across Landscape and Vertical Strata in Indian River County, Florida. 2021. Pathogens, 10:1575–1587. https://doi.org/10.3390/pathogens10121575
Graham M. 1906. Four new Culicidae from Jamaica, West Indies. 1906. The Canadian Entomologist, 58:311–320. https://doi.org/10.4039/Ent38311-9
Henry CJ, Pillai AN, Lednicky JA, Morris JG Jr, Hladish TJ. 2022. Ecology and public health burden of Keystone virus in Florida. Epidemics, 39:100555. https://doi.org/10.1016/j.epidem.2022.100555
Howard LO, Dyar HG, and Knab F. 1917. The mosquitoes od North and Cental America and the West Indies. Carnegie Institution of Washinton, Washington DC, Vol. 4. Part II P. 618–619, 790–794.
Integrated Taxonomic Information System (ITIS) 2021. Report: Aedes pertinax Grabham, 1906 (Taxonomic Serial No.: 1154548). Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=1154548#null. (Accessed on 30 June 2022).
Knight KL and Stone A. 1977. A catalog of the mosquitoes of the world (Diptera: Culicidae). 2nd ed. Annapolis, MD. Thomas Say Foundation, Entomological Society of America, Vol. 6, P.1–611.
Komp WHW. 1949. Aedes (Ochlerotatus) nubilus Theobald, 1903 a synonym of Aedes (Ochlerotatus) serratus Theobald, 1901. Aedes (Ochlerotatus) nubilus Theobald, 1903 un sinónimo de Aedes (Ochlerotatus) serratus Theobald, 1901. Proceedings of the Entomological Society of Washington, 51:105–115.
Reinert JF. 2000. New classification for the composite genus Aedes (Diptera: Culicidae: Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. Journal of the American Mosquito Control Association-Mosquito News, 16:175–188.
Reeves LE, Medina J, Miqueli E, Sloyer KE, Petrie W, Vasquez C, Burkett-Cadena ND. 2021. Establishment of Aedes (Ochlerotatus) scapularis (Diptera: Culicidae) in Mainland Florida, With Notes on the Ochlerotatus Group in the United States. Journal of Medical Entomology. 58:717–729. https://doi.org/10.1093/jme/tjaa250
Riles MT and Connelly CR. 2020. An update of the mosquito records in Florida Counties, USA. Journal America Mosquito Control Association, 36:107–111. https://doi.org/10.2987/20-6923.1
Rueda J and María Alarcón-Elbal PM. 2020. Mosquitos (Diptera: Culicidae) de lagunas temporales de Costa Rica y Nicaragua Limnetica, 39:579–600. DOI: 10.23818/limn.39.38.
Sloyer KE, Santos M, Rivera E, Reeves LE, Carrera JP, Vittor AY, Valderrama A, and Burkett-Cadena ND. 2022. Evaluating sampling strategies for enzootic Venezuelan equine encephalitis virus vectors in Florida and Panama. PLoS Neglected Tropical Diseases, 13;16:e0010329. https://doi.org/10.1371/journal.pntd.0010329
Shroyer D, Harrison BA, Bintz BJ, Wilson M, Sither CB and Byrd BD. 2015. Aedes Pertinax, a newly recognized mosquito species in the United States. Journal of the American Mosquito Control Association, 31:97–100. https://doi.org/10.2987/14-6447R.1
Theobald FV. 1901. A monograph of the Culicidae or mosquitoes. Vol 3. British Museum, London UK, Vol. 2, P. 37, 44. Available online: https://mosquito-taxonomic-inventory.myspecies.info/monograph-culicidae-or-mosquitoes-0 (Accessed on 26 July 2022).
Theobald FV. 1903. A monograph of the Culicidae or mosquitoes. Vol 3. British Museum, London UK, Vol. 3 P. 359. Available online: (Accessed on 26 July 2022).
Theobald FV. 1907. A monograph of the Culicidae or mosquitoes. Vol 4. British Museum, London UK, Vol. 7, P. 465.
Tyler-Julian K, Reeves Lawrence, Lloyd A, Hoel David. 2022. Aedes pertinax and Culex interrogator: two mosquito species new to Lee County, Florida. Journal of the Florida Mosquito Control Association, 69:16–20. https://doi.org/10.32473/jfmca.v69i1.130621
Walter Reed Biosystematics Unit (2021). Aedes atlanticus species page. Walter Reed Biosystematics Unit Website, http://wrbu.si.edu/vectorspecies/mosquitoes/atlanticus. (Accessed on 3 June 2022).