The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
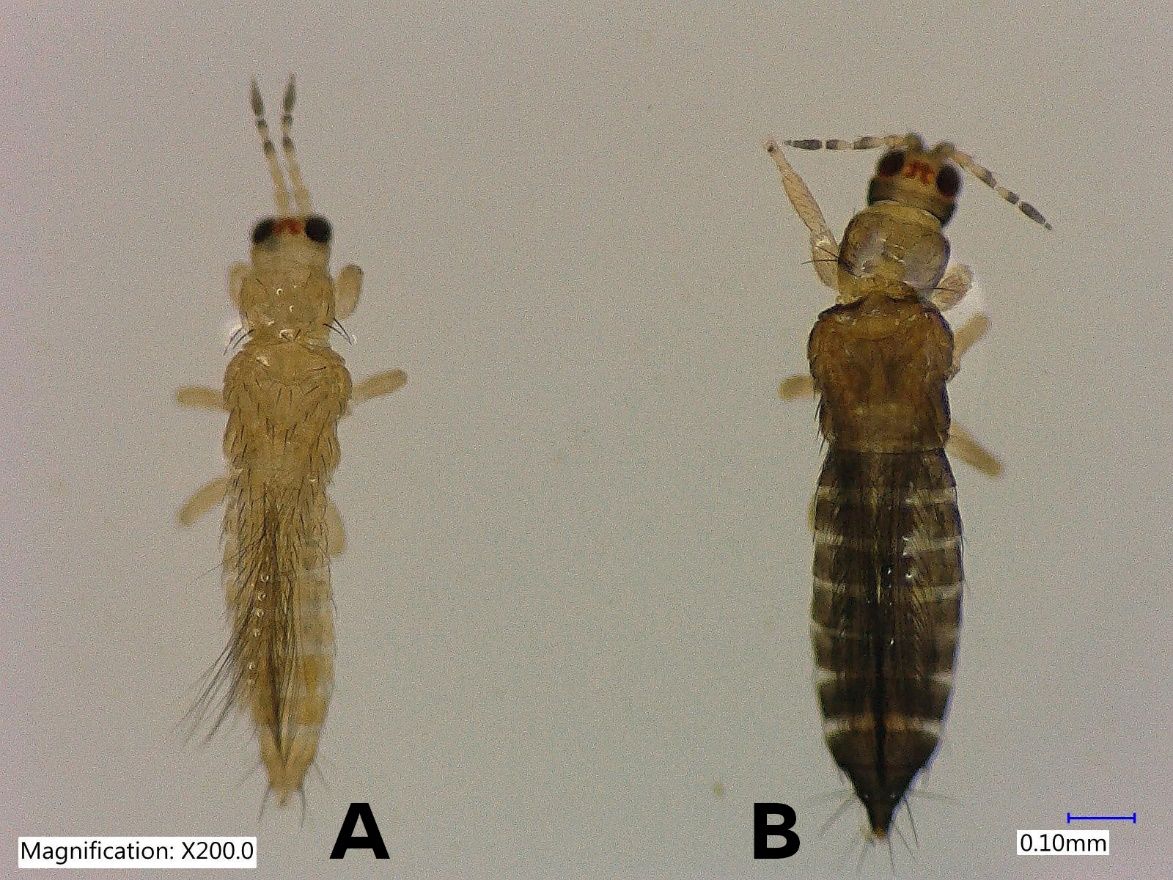
Thrips parvispinus (Karny, 1922) (Figure 1) is a member of the “Thrips orientalis group” (Mound 2005). Thrips parvispinus is commonly known as Taiwanese thrips or Southeast Asia thrips (Shridhar et al 2021). Thrips parvispinus was identified as a serious pest of several plant species in Southeast Asia and a species of quarantine importance. Thrips parvispinus was reported in 2020 for the first time in the United States in Orange County, Florida (Soto-Adames 2020).

Credit: A. Khan, UF/IFAS Entomology and Nematology Department
Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
Synonymy
Isoneurothrips parvispinus Karny, 1922
Isoneurothrips jenseni Karny, 1925
Isoneurothrips pallipes Moulton, 1928
Thrips (Isoneurothrips) taiwanus Takahashi, 1936
Taxonomic Position
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Thrips Linnaeus, 1758
Species: Thrips parvispinus (Karny, 1922)
Distribution
Thrips parvispinus is native to the Asian tropics and has been reported from Indonesia, Thailand, Malaysia, Singapore, Taiwan (Mound and Masumoto 2005), Yunnan-China (Zhang et al. 2011), the Philippines (Reyes 1994), Australia, the Solomon Islands (Palmer 1992), India (Tyagi 2015; Rachana et al. 2018), and New Zealand (Moritz et al. 2013). In Africa, T. parvispinus was found in the French overseas department of La Réunion (Bournier 2000), Mauritius (Mound 2010), and the mainland in Tanzania (Dar-el Salaam) and Uganda (Kampala) (Moritz et al. 2013). In Europe, Thrips parvispinus has been reported in Greece (Mound and Collins 2000), Spain (Lacasa et al. 2019), and France (EFSA 2019). Thrips parvispinus was recorded in Hawaii in 2006 (Sugano et al. 2013). Recently, Thrips parvispinus was reported from Barbados in the Caribbean and in Orange County, Florida, in the continental United States (Soto-Adames 2020).
Identification
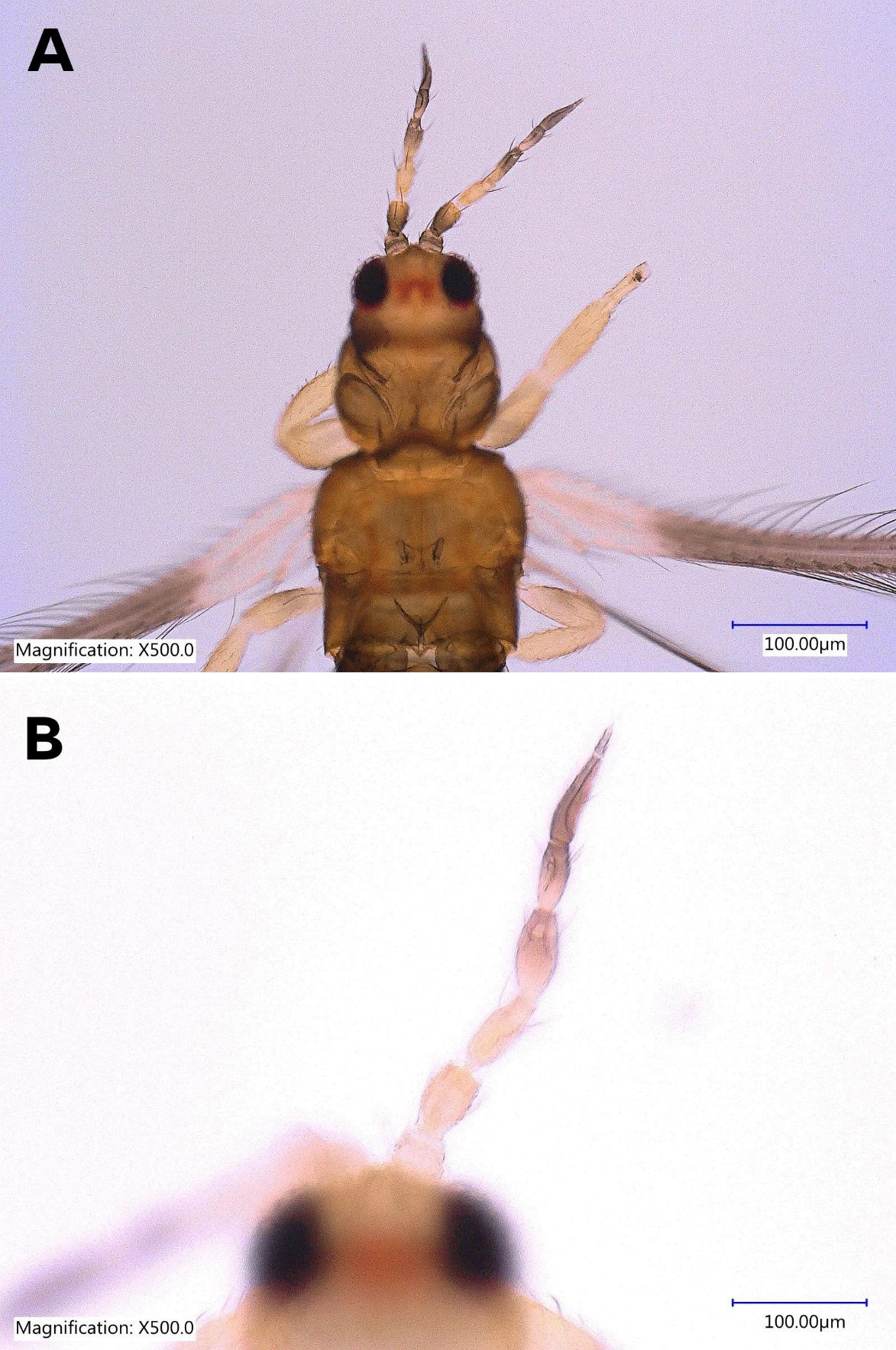
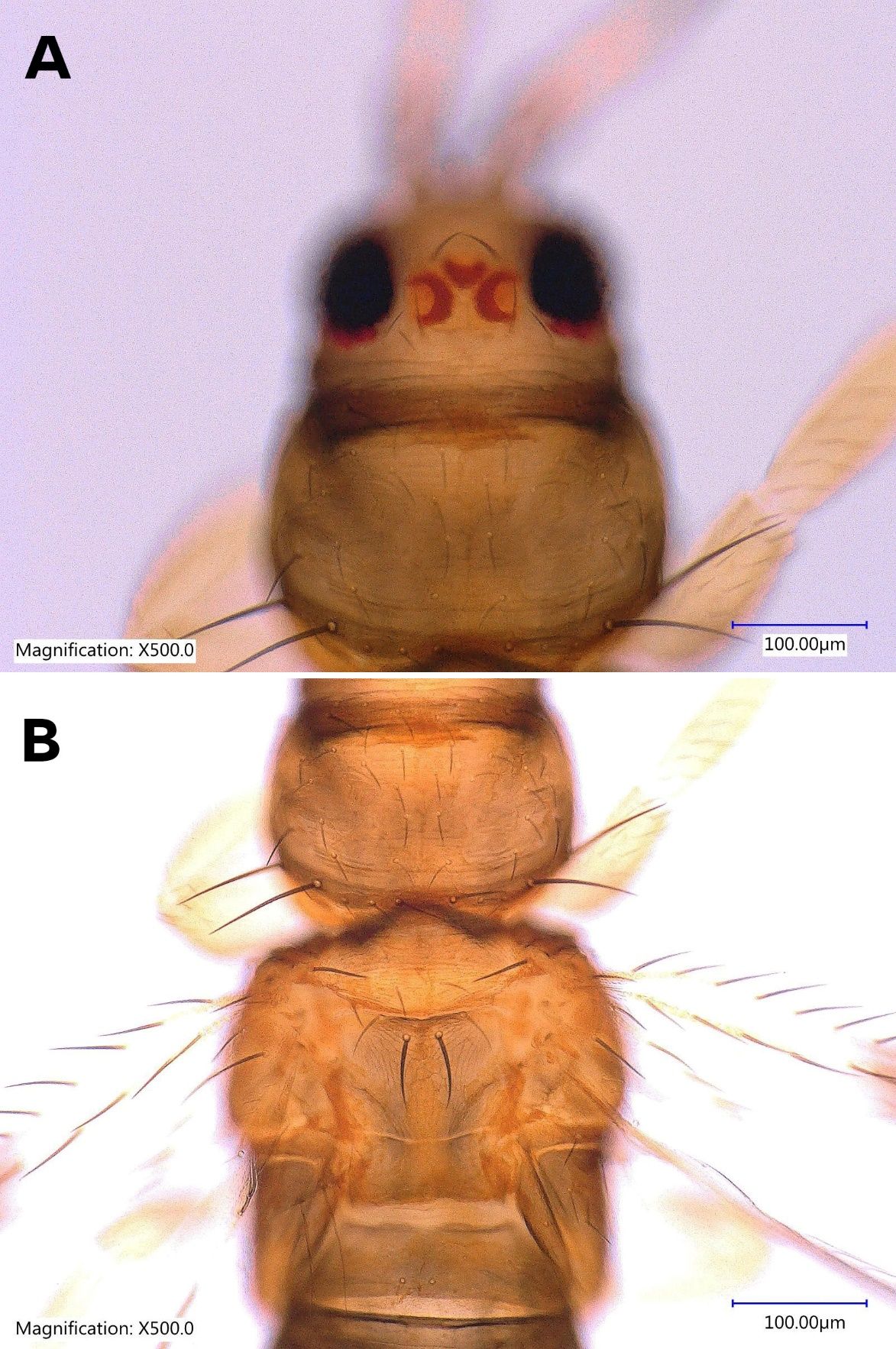
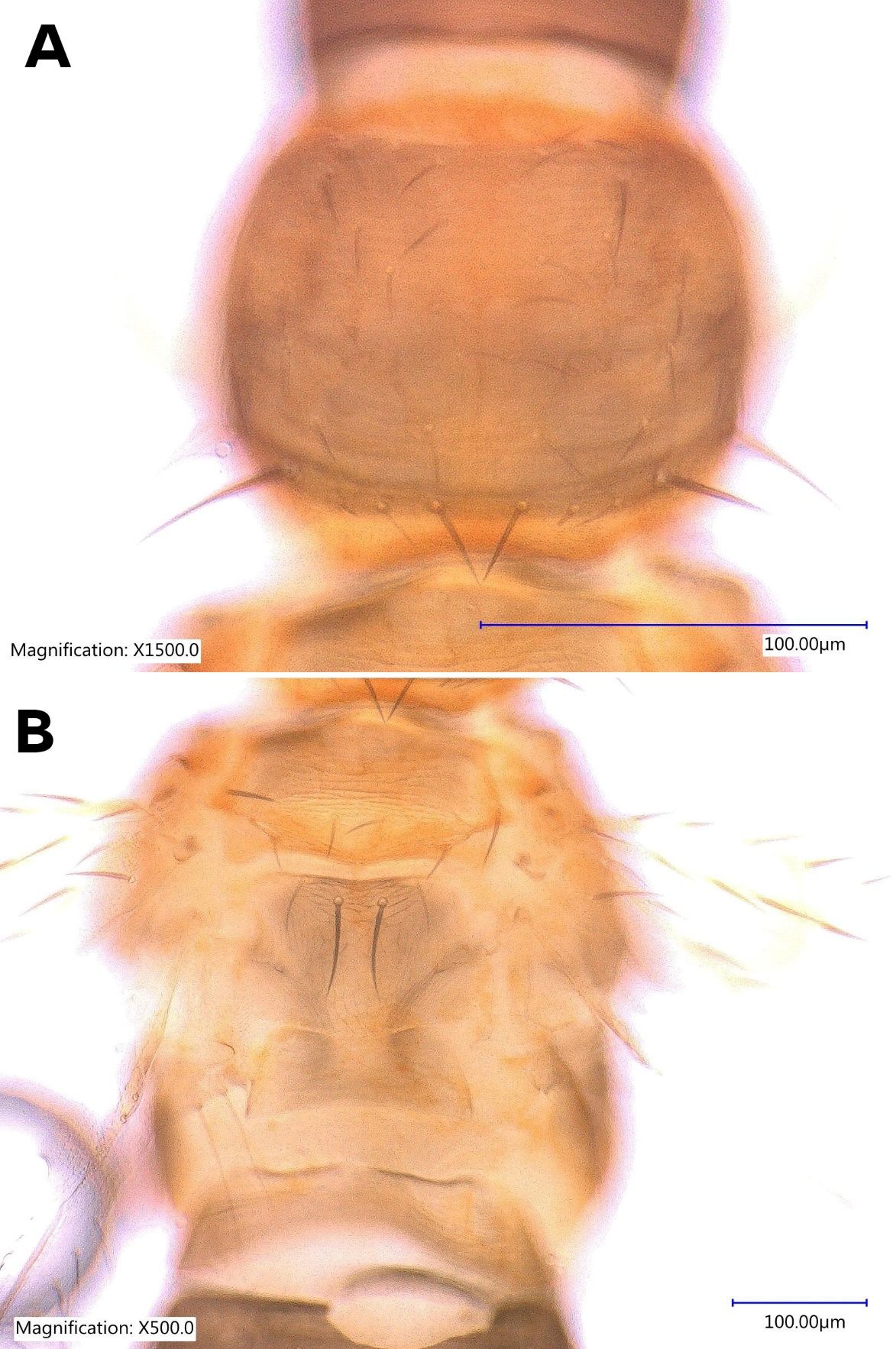
The female Thrips parvispinus is dark brown, with the head and thorax paler than the abdomen (Figure 1A, Figure 2). The legs are yellow. Head is one and a half times as wide as long, cheeks arched darker than the median area. Antennal segment III and the basal half of IV–V are yellow. Forewings are brown with a base sharply pale. Antennae are 7-segmented (Figure 3), III–IV constricted at apex, VII small. Head is wider than long, ocellar setae pair III small and arising on anterior margins of the triangle (Figure 4A); postocular setae pairs I and III slightly longer than ocellar setae III, pair II minute. Posterior margin of the pronotum has 3 pairs of setae. Metanotum is reticulate medially, reticles varying in shape and sometimes with faint internal markings; median setae long and arising behind anterior margin; campaniform sensilla absent (Figure 5A and 5B). First and second veins of fore wings with complete rows of setae; clavus with 5 marginal setae (Figure 6). Tergite II with 3 lateral marginal setae; posterior margin of tergite VIII with comb absent, a few microtrichia present laterally (Figure 7A and 7B); pleurotergites without discal setae. Sternites II & VII without discal setae, III–VI with about 6 to 12 discal setae in an irregular row. Both female and male Thrips parvispinus are macropterous (i.e., with long or large wings).
The male Thrips parvispinus is yellow (Figure 1 B and Figure 2). There is no posteromarginal comb on the tergite VIII (Figure 8). The sternites III–VII have a small transverse pore plate while discal setae arise laterally (Karny 1922).
Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department

Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
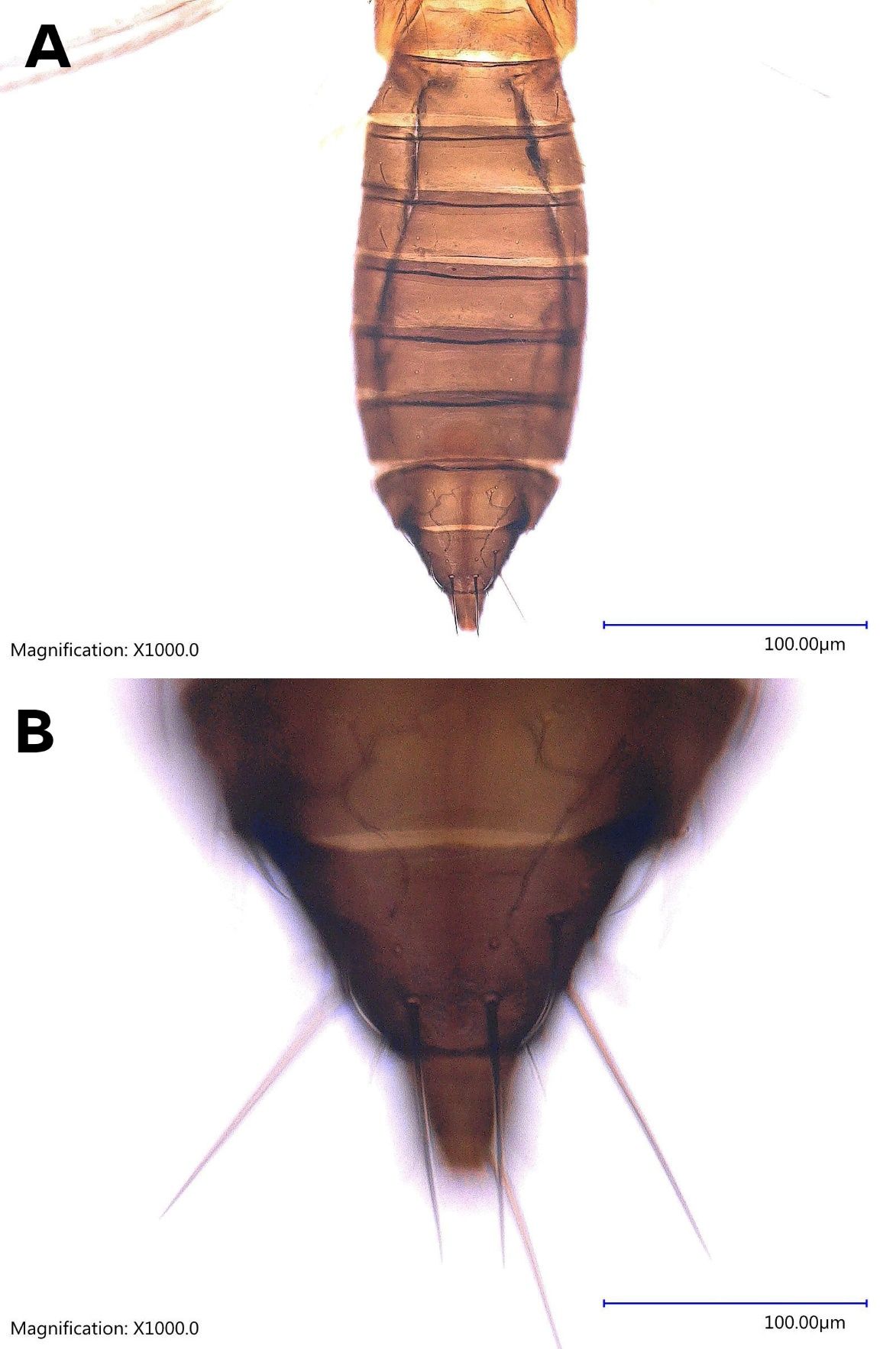
Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
Related Species
Thrips orientalis: Sternites lll–Vl with 6-13 discal setae in an irregular row.
Thrips australis, Thrips microchaetus, Thrips subnudula and Thrips tenellus: sternites lll–Vll with at least 1 pair of discal setae and pleurotergites with discal setae.
Thrips acacia, Thrips brevisetosus, Thrips florum, Thrips gowdeyi, Thrips hawaiiensis and Thrips simplex: sternites lll–vll with at least 1 pair of discal setae and pleurotergites without discal setae.
Thrips nigropilosus, Thrips palmi, Thrips pusillus and Thrips tabaci: sternites and pleurites without discal setae
Potential to Spread and Species Replacement Capabilities
Thrips parvispinus originated from Thailand and was spread through international trade of ornamental and vegetable materials to other countries including Indonesia, Thailand, Cambodia, and Vietnam (Mound and Collins 2000, Vos 1994, Murai 2002, Sartiami and Mound 2013, Johari 2015). Thrips parvispinus was intercepted in the Netherlands in 1996 from shipments of cut flowers from Indonesia and Asia. Thrips parvispinus also arrived in the Netherlands through Ixora sp. potted plants from Thailand in 2005 and through a Whrightia sp. potted plant from Indonesia in 2013. It was intercepted in the UK from Gardenia sp. from Indonesia (Mound and Collins 2000), and through orchid plants from Malaysia (Collins 2010). In Switzerland, Thrips parvisinus was reported to come from Rosa spp. from Thailand, Momordica charantia from Sri Lanka and a vegetable shipment of Solanum aethiotipum from Uganda (EPPO, 2016). Thrips parvispinus arrived in France on Momordica charantia from Cambodia (EPPO 2014). In Japar, Thrips parvispinus was first recorded on Heliconia sp. from Mauritius (Masumoto et al. 2003). Thrips parvispinus was found to replace Thrips palmi Karny populations, which was the major thrips pest of vegetable crops in Indonesia (Murai et al. 2010). Sridhar et al. (2021) observed that the population of Thrips parvispinus displaced the well-established chili thrips, Scirtothrips dorsalis Hood, population in chili (Capsicum frutescens L.) ecosystems in Andhra Pradesh, Karnataka, and Telengana States of India. Thrips pavispinus invaded Florida in 2020 (Soto-Adames 2020).
Life Cycle and Biology
Thrips parvispinus reproduces sexually and life stages consist of an egg, two larval stages, a prepupa, a pupa, and an adult. The female prefers to oviposit on young leaves and flowers. Hutasoit et al. (2017) observed the biology of Thrips parvispinus on chili pepper leaves. The female Thrips parvispinus lays 15.33 eggs on average. The incubation period is four to five days after the female inserts eggs into the leaves. The duration of different life stages is 4.79 days for egg, 1.36 days for first larval instar, 3.54 days for second larval instar, 1.08 days for prepupa, and 1.96 days for pupa. The longevity of females and males is 8.55 days and 6.00 days, respectively. The preoviposition period of Thrips parvispinus is 1.11 days. Thrips parvispinus can complete their life cycle within 13.68 days. The intrinsic rate of increase of Thrips parvispinus was 0.15 individuals per day per female; the net reproductive rate was 5.71 individual per female per generation. The generation time of Thrips parvispinus was found to be 11.49 days and the doubling time was 4.57 days. The mean fecundity and mean generation time of Thrips parvipinus at 20°, 25°, and 30° C were 50, 69, and 56 eggs, and 37.6, 24.8, and 18.8 days, respectively (Hutasoit et al. 2017). The intrinsic rate of natural increase of Thrips parvispinus was 0.18, 0.24, and 0.37 at 20°, 25°, and 30° C, respectively. There can be several generations of Thrips parvispinus in a year depending on environmental factors (Murai et al. 2010). The highest flight activity of adult Thrips parvispinus was found at 9:00 - 10:00 and the lowest was at 18:00 - 19:00 (6:00 - 7:00pm). The larval stages of Thrips parvispinus were more abundant on leaves while the adults were more abundant in flowers (Pratiwi et al. 2018).
Hosts
Thrips parvispinus is a polyphagous species and has a wide host range including fruits, vegetables, and ornamental plants (EPPO 2001, Masumoto et al. 2003, EPPO, 2016, Azidah 2011, Moritz et al. 2013, Tyagi et al. 2015, Hutasoit et al. 2017, NPPO 2019, Nagaraju et al. 2021, Johari et al. 2014, Sridhar et al. 2021, Prasannakumar et al. 2021) listed below.
Table 1.
The host preference of Thrips parvispinus varies with its geographical distribution. In the established region of Thrips parvispinus, papaya, peppers, potatoes, eggplant, beans, shallots, and strawberries were reported as the most affected crops.
Injury
Host plant injury is caused by feeding and breeding on young leaves and flowers. The symptoms of injury include deep punctures and scratches on the underside of leaves, reddish brown leaf undersides and yellowish tops, necrotic areas on distorted leaf lamina with yellow streaking, brownish streaks on petals, drying and withering of flowers, and affected fruit set. Thrips parvispinus affects plant growth because of feeding on growing portions of the plant (Sireesha et al. 2021). Infested leaves showed irregular color and streak lines (Moritz et al. 2013) followed by leaf curling and deformation.
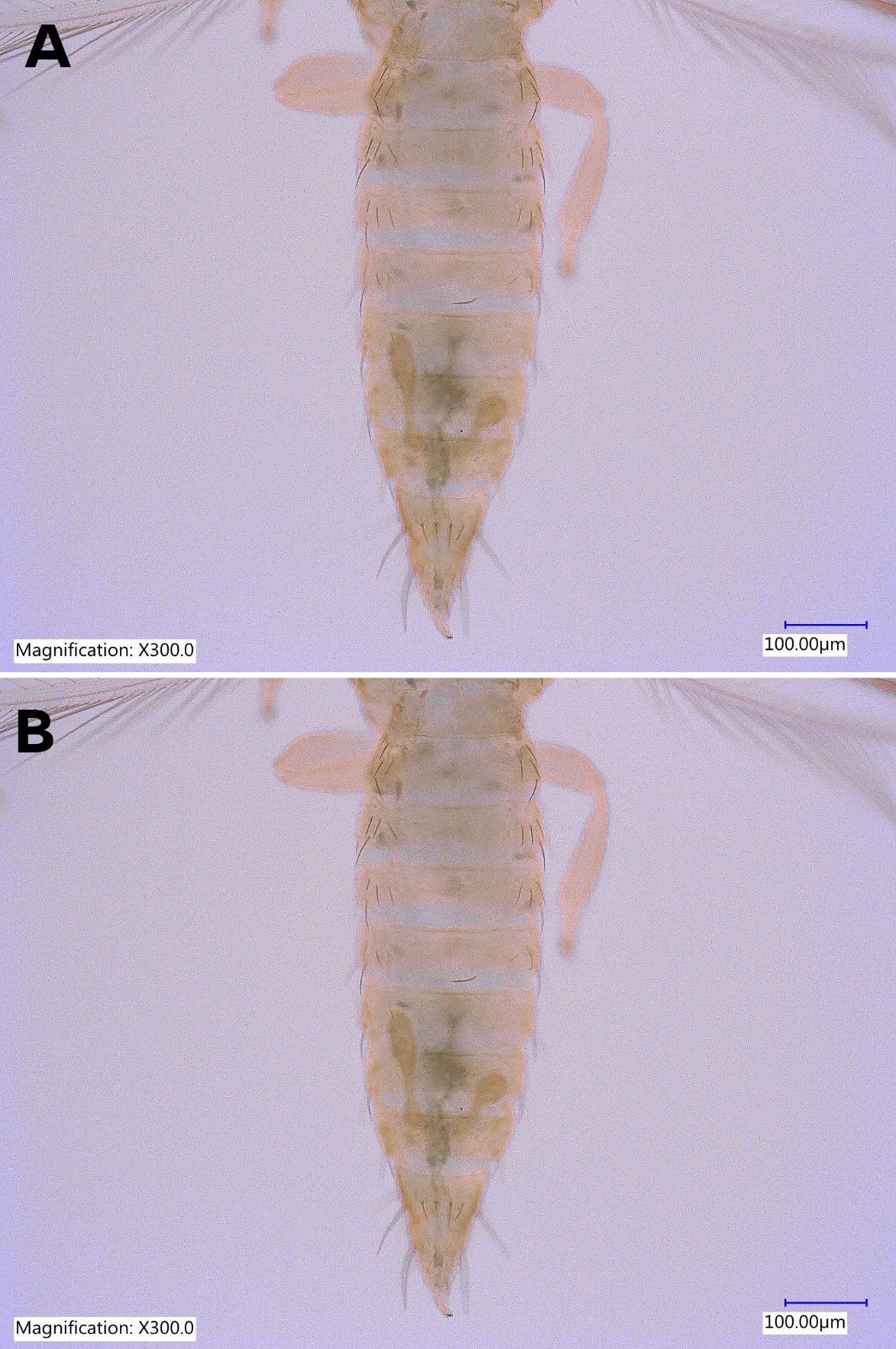
Thrips parvispinus is a serious pest of chili pepper (Vos and Frinking 1998) in Indonesia and has replaced melon thrips (Thrips palmi Karny) on various vegetable crops (Murai et al. 2010). Feeding damage caused scarring of fruits and foliage of papaya in Hawaii (Sugano et al. 2013). In Florida, feeding injury of Thrips parvispinus was observed on Anthurium leaves, Hoya buds, and Gardenia sp. leaves (Figure 9A and 9B) at nursery facilities.

Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
Disease transmission
The feeding damage of Thrips parvispinus has been observed to be associated with the saprophytic fungus Cladosporium oxysporum Berk. & M. A. Curtis on papaya fruit cause bunchy-top disease (malformed leaves and shot holes on new flushes) (Lim 1989). Thrips parvispinus has been reported as a vector of tobacco streak virus, a pollen-borne plant virus transmitted by thrips while feeding (Klose et al. 1996). There is no report of Thrips parvispinus as a vector tospovirus.
Quarantine significance
Thrips parvispinus originated in intensive agriculture and acquired the habit of polyphagy, spreading in diverse habitats. It is related to western flower thrips (Frankliniella occidentalis Pergande) and melon thrips (Thrips palmi Karny) which also originated in agriculture-rich intensive horticulture and emerged as international pests of economic importance (Mound and Collins 2000). Thrips parvispinus feeds on various horticultural plants and vegetables which have trading value. Through these host plants, Thrips parvispinus could extend its distribution. A strong quarantine action is warranted to limit its movement between the states in the United States.
Management
Sampling
White sticky traps attracted more Thrips parvispinus than blue or yellow traps (Murai et al. 2010). The survey of constant systematic monitoring and inspection is urgent in newly infested areas with susceptible crops (Sridhar et al. 2021). Shireesha et al (2021) reported that the establishment of blue and yellow sticky traps at a density of 30 traps per acre can be helpful to monitor Thrips parvispinus as well as to reduce adult populations in chili field (Sireesha et al. 2021).
Cultural Control
The use of healthy and pest-free seedlings, removal of infested plants from the field, and destruction of highly infested fields can reduce the future spread (Sridhar et al. 2021). Avoiding establishing crops near any infected hosts and destroying weed and wild hosts near the crop field can reduce infestations. Higher Thrips parvispinus populations were observed in coriander flowers. Thus, coriander can be used as a trap crop around the highly infested crops (Prasannakumar et al. 2021). Sridhar et al. (2021) recommended avoiding excessive nitrogen fertilizer. In addition, split fertilizer (nitrogen and potash) applications during crop growth can be helpful to reduce Thrips parvispinus incidence in the susceptible crops (Sireesha et al. 2021, Sridhar et al. 2021). A resistant line of chili pepper has been identified against Thrips parvispinus (Maharijaya et al. 2011).
Chemical Control
Laboratory studies revealed that spinosad is effective in controlling T. parvispinus but not acetamiprid (Murai et al. 2010). Prassanakumar et al. (2021) observed that rotation with biopesticides such as Beauveria bassiana or Lecanicillium lecanii, Arka neem and ponagamia soaps, and neem oil with insecticidal sprays such as Spinosad 45SC can reduce the pest population in chili. Rotation of insecticides with a different mode of action can reduce the probability of insecticide resistance in Thrips parvispinus. Targeting sprays at flowers and growing buds can be used as efficient use of chemicals as Thrips parvispinus lives and feeds on them (Sugano et al. 2013). When there is a Thrips parvispinus outbreak, rotation of insecticides viz., fipronil, fipronil + imidacloprid, cyantraniliprole, acetamiprid, and spirotetramat can be used (Sireesha et al., 2021). Sequential spray with fipronil or cyantraniliprole or acetamiprid or spinosad water at weekly intervals can be effective to manage Thrips parvispinus (Anitha Kumari et al. 2021). However, heavy insecticide exposure in chili, the most susceptible crop, can result in the resurgence of Thrips parvispinus (Sireesha et al. 2021). Thus, growers need to follow the label rate to use any chemical insecticides and fertilizers, and follow the local regions/Universities/Departments recommendation (ICAR-NBAIR, Pest Alert, 2021).
Biocontrol Agents
Ladybird beetles, Menochilus sexmaculatus Fabricius and Coccinella transversalis Fabricius, and entomopathogenic fungus Lecanicillium lecanii R. Zare & W. Gams are potential natural enemies of Thrips parvispinus. The use of M. sexmaculatus and L. lecanii was as effective in controlling Thrips parvispinus and increasing the yield of the crop (Prabaningrum et al. 2008). Microbial biopesticides such as Pseudomonas fluorescence NBAIRPFDWD or Bacillus albus NBAIR-BATP are also effective in suppressing Thrips parvispinus when sprayed on flowers and fruits (Sridhar et al. 2021).
Conclusion
Thrips parvispinus is a cosmopolitan species and a species of pest significance. Thrips parvispinus is polyphagous and exposure to heavy insecticide applications revealed characteristics comparable to those of Frankliniella occidentalis and Thrips palmi, which are some of the most important pests of cultivated crops worldwide. It is important to establish strong monitoring capabilities to evaluate the presence of Thrips parvispinus in the imported crops, grains, cut flowers, ornamentals, and fruits. Thrips parvisponus is an invasive pest of concern for the Florida nursery industry. Though Thrips parvispinus is now confined to Florida in the USA, proper management should be implemented to mitigate the spread of this species to other states.
Acknowledgment
The information about the invasion of Thrips parvispinus in Barbados was brought to our attention by Dr. Matthew Ciomperlik, Laboratory Director, USDA-APHIS-PPQ-S & T Mission and Phoenix Laboratories. Dr. Ciomperlik also provided information about Thrips parvispinus damage potential and quarantine significance.
Selected References
Azidah AA. 2011. Thripidae (Thysanoptera) species collected from common plants and crops in Peninsular Malaysia. Scientific Research and Essays 6(24): 5107-5113.
Bournier JP. 2000. Thysanopteres de lile de la Reunion: Terebrantia, Bulletin de la Societe entomologique de France 105: 65-108.
Collins DW 2010. Thysanoptera of Great Britain: A revised and updated checklist. Zootaxa 2412(2412):21-41. DOI: 10.11646/zootaxa.2412.1.2.
EPPO 2001. Mini data sheet on Thrips parvispinus (Thysanoptera: Thripidae) - A south-east Asian thrips. https://gd.eppo.int/download/doc/1100_minids_THRIPV.pdf [accessed July 31, 2019].
EPPO Reporting Service no. 05 – 2014. EPPO report on notifications of non-compliance. 2014/091. https://gd.eppo.int/reporting/article-2811 [accessed July 31, 2019].
EPPO Reporting Service no. 10 – 2016. EPPO report on notifications of non-compliance. 2016/183. https://gd.eppo.int/reporting/article-5928 [accessed July 31, 2019].
Hutasoit RT, Triwidodo H, Anwar R. 2017. Biology and demographic statistic of Thrips parvispinus Karny (Thysanoptera: Thripidae) in chili pepper (Caspicum annuum Linnaeus). Indonesian Journal of Entomology, 14: 107-116.
ICAR-NBAIR. Pest Alert: Invasive thrips, Thrips parvispinus (Karny) threatening chilli cultivation in India.
Johari A, Herlinda S, Pujiastuti Y, Irsan C, Sartiami D. 2014. Morphological and genetic variation of Thrips parvispinus (Thysanoptera: Thripidae) in chilli plantation (Caspicum annuum L.) in the lowland and highland of Jambi Province, Indonesia. American Journal of Bioscience 2: 17-21.
Karny H. 1922. Thysanoptera from Siam and Indo-China. Journal of the Siam Society 16(2): .91-153.
Klose MJ, R. Sdoode R, Teakle DS, Milne JR, Greber RS. 1996. Transmission of three strains of Tobacco streak ilavirus by different thrips species using virus-infected pollen. Journal of Phytopathology 144: 282-284. Doi: https://doi.org/10.1111/j.1439-0434.1996.tb01530.x
Lacasa A, Lorca M, Martinez MC, Bielza P, Guirao P. 2019. Thrips parvispinus (Karny, 1922), un nuevo trips en cultivos de plantas ornamentales. Phytoma Espana 311: 62-69.
Lim WH. 1989. Bunchy and malformed top of papaya cv. Eksotika caused by Thrips parvispinus and Cladosporium oxysporum. MARDI Research Journal, 17(2): 200-207 (abst).
Lemmet S. 2019. Ce qu'il faut retenir. Thrips. Bulletin de Santé Végétal. Grand Sud-Ouest. Edition Horticulture 1: 6-9. http://draaf.occitanie.agriculture.gouv.fr/IMG/pdf/bsv_na_horti_gso_1_20190513_cle03f5e8.pdf
Maharijaya A, Vosman B, Steenhuis-Broers G, Harpenas A, Purwito A, Visser RGF, Voorrips RE. 2011. Screening of pepper accessions for resistance against two thrips species (Frankliniella occidentalis and Thrips parvispinus). Euphytica 177: 401-410.
Mound LA. 2010. Species of the genus Thrips (Thysanoptera, Thripidae) from the Afro-tropical region. Zootaxa 2423: 1-24.
Mound LA, Masumoto M. 2005. The genus Thrips (Thysanoptera, Thripidae) in Australia, New Caledonia and New Zealand, Zootaxa 1020: 1-64.
Moritz G, Brandt S, Triapitsyn S, Subramanian S. 2013. Identification and information tools for pest thrips in East Africa. CBIT Publishing, Queensland. Thrips parvispinus (Karny, 1922). http://thripsnet.zoologie.uni-halle.de/key-server-neu/data/0a0b0a0e-0d03-4106-8306- 08060a080902/media/Html/Thrips%20parvispinus.html [accessed August 1, 2019].
Mound LA, Collins DW 2000. A southeast Asian pest species newly recorded from Europe: Thrips parvispinus (Thysanoptera: Thripidae), its confused identity and potential quarantine significance. European Journal of Entomology 97: 197-200.
Murai T. 2002. The pest and vector from the East: Thrips palmi. Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera; Canberra: Australian National Insect Collection; 2002. pp. 19–32.
Murai T, Watanabe H, Foriumi W, Adati F, Okajima S. 2010. Damage to vegetable crops by Thrips parvispinus Karny (Thysanoptera: Thripidae) and preliminary studies on biology and control. Journal of Insect Science 10(141): 27–28. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3388970/ [accessed July 29, 2019].
Nagaraju, DK, Uppar V, Ranjith M, Sriharsha Ramesh G, Verma OM, Prakash R. 2021. Occurrence of Thrips parvispinus (Karny) (Thripidae: Thysanoptera) in major chilli (Capsicum annum) growing areas of Karnataka. Insect Environment 24(4): 523-532.
NPPO, 2019. Thrips parvispinus. Quick scan, QS.Ent./ 2019/001.
Palmer JM. 1992. Thrips (Thysanoptera) from Pakistan to the Pacific: a review. Bulletin of the British Museum Natural History (Entomology). 61(1): 1-76.
Prabaningrum L, Moekasan TK, Udlarto BK, den Belder E, Ellings A. 2008. Integrated pest management on sweet pepper in Indonesia: Biological control and control thresholds for thrips. Acta Horticulturae (ISHS). 767: 201-210.
Prasannakumar N, Venkataravanappa, V, Rachana R, Sridhar V, Govindappa M, Basavarajappa M, Hemalatha K, Aswathnarayana D, Reddy MK, Samuel D. 2021. Status of the outbreak of Thrips parvispinus (Karny) on chilli in Karnataka. Pest Management in Horticultural Ecosystems 27(2): 286-290.
Pratiwi NIPE, Supartha IW, Yuliadhi DKA. 2018. Flight activities and population development of Thrips parvispinus Karny (Thysanoptera: Thripidae) on chili (Capsicum annuum L.). Agrotrop 8(1): 28-36.
Rachana RR, Roselin P, Varatharajan R. 2018. Report of invasive thrips species, Thrips parvispinus (Karny) (Thysanoptera: Thripidae) on Dahlia rosea (Asteraceae) in Karnataka. Pest Management in Horticultural Ecosystens 24(2): 175-176.
Reyes CP. 1994. Thysanoptera (Hexapoda) of the Philipine Islands. The Raffles Bulletin of Zoology 42: 1-507.
Sartiami D, Mound LA. 2013. Identification of the terebrantian thrips (Insecta, Thysanoptera) associated with cultivated plants in Java, Indonesia. ZooKeys. 2013;306:1–21. doi: 10.3897/zookeys.306.5455.
Seki M, Murai T. 2012. Responses of five adult thrips species (Thysanoptera; Thripidae) to high carbon dioxide atmospheres at different temperatures. Applied Entomology and Zoology 47(2): 125-128.
Sireesha K, Prasanna BVL, Vijaya Lakshmi T, Reddy RVSK. 2021. Outbreak of invasive thrips species Thrips parvispinus in chilli growing areas of Andhra Pradesh. Insect Environment 24(4): 514-519.
Soto-Adames FN. 2020. Thrips parvispinus (Karny). Pest Alert, Florida Department of Agriculture and Consumer Services, Division of Plant Industry, FDACS-P-01926.
Sridhar V, Chandana PS, Rachana R. 2021. Global status of Thrips Parvispinus (Karny, 1922), an invasive pest. The Journal of Research PJTSAU 49(4): 1-11.
Sugano J, Hamasaki R, Villalobos E, Chou M, Wright M, Fukuda S, Swift S, Ferreira S, Tsuda D, Derval, Diaz-Lyke C, Nakamoto ST. 2013. Damage to Papaya caused by Thrips parvispinus (Karny). [poster] https://www.ctahr.hawaii.edu/oc/freepubs/pdf/Papaya_Thrips_poster.pdf [accessed July 29, 2019].
Tyagi K, Kumar V, Singha D, Chakraborty R. 2015. Morphological and DNA barcoding evidence for invasive pest thrips, Thrips parvispinus (Thridae: Thysanoptera), newly recorded from India. Journal of Insect Science 15(1): 105.
Vos JGM 1994. Integrated crop management of hot pepper (Capsicum spp.) in tropical lowlands. 1994. p. 188. PhD Thesis, Wageningen Agricultural University, the Netherlands.
Vos JGM, Frinking HD. 1998. Pests and diseases of hot pepper (Capsicum spp.) in tropical lowlands of Java, Indonesia. Journal of Plant Protection in the Tropics, 11(1): 53-71.
Zhang HR, Xie YH, Li ZY. 2011. Identification key to species of Thrips genus from China (Thysanoptera, Thripidae), with seven new records. Zootaxa 2810: 37-46, DOI: 10.5281/zenodo.277122.