Introduction
This publication includes information blueberry growers should consider when selecting fungicides and insecticides to apply during bloom and application strategies to minimize harm to pollinators. Insect pollinators, particularly wild and managed bees, are necessary to achieve adequate fruit set and marketable berries in southern highbush blueberry production. Bees facilitate both self-pollination, including pollination within an individual flower or bush, and cross-pollination, or that between bushes of different cultivars. In the absence of insect pollinators, berries may form, but they will be significantly smaller and misshapen and will take longer to ripen than bee-pollinated berries (Danka et al. 1993; Campbell et al. 2018; Mallinger et al. 2021). For these reasons, insect pollinators are essential to the production of marketable and profitable southern highbush blueberries.
Blueberry growers in Florida typically stock their fields with honey bees (Apis mellifera) as well as managed bumble bees (Bombus impatiens) (Mallinger et al. 2021). Wild insect pollinators, including the native southeastern blueberry bee (Habropoda laboriosa), native carpenter bees (Xylocopa spp.), and native butterflies and wasps, also contribute to pollination (Campbell et al. 2018; Mallinger et al. 2021; Rogers et al. 2014). Both managed and wild pollinators are susceptible to pesticide applications, especially when those applications occur during the bloom period when pollinators are actively foraging in blueberry fields. Growers must balance disease and pest protection and adequate insect pollination.
How are insect pollinators exposed to pesticides?
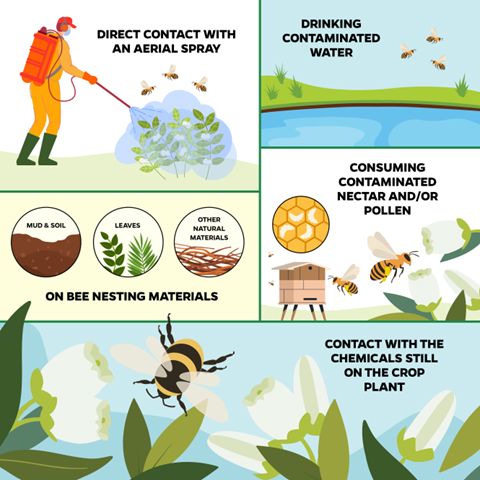
Pollinators can be exposed to pesticides in several ways (Figure 1), including those listed below:
- Direct contact with an aerial spray: most likely to happen if the pesticide is sprayed during the day and in favorable weather for pollinator activity (warm and relatively sunny).
- Contact with the chemical while it is still active on the crop plant during the period of residual activity: most likely to happen when pesticides are applied in the daytime and during weather favorable for pollinator activity. This also includes contact with pesticide residues on flowering weeds within the crop field or in the vicinity of the crop field.
- Drinking contaminated water: this is particularly an issue when pesticides are applied via irrigation. If there are leaks in the drip irrigation system, or if water pools in low areas of the field, bees may drink the contaminated water. Pesticide residues from other modes of application may also be present in surface or groundwater.
- Consuming contaminated nectar and/or pollen: this is particularly an issue for systemic products. If the pesticide is systemic (i.e., taken up by the plant and expressed throughout the plant tissues), it may be present in the nectar and pollen of the crop plant even well after application. Though the concentration of the pesticide within nectar or pollen is often relatively low, consuming contaminated pollen or nectar can have sublethal effects on adult bees or lethal effects on the bee brood (Yang et al. 2008; Whitehorn et al. 2012; Stoner and Eitzer 2012).
- Via bee nesting materials, including soil, mud, leaves, and other natural materials. Wild bees use a variety of natural materials to create their nests. Contaminated soil, leaves, or other materials can harm wild bee larvae living and growing in these nests. Systemic insecticides have proven to be highly mobile, which increases the likelihood that they will contaminate nesting materials (Goulson 2013; Main et al. 2014; Long and Krupke 2016).
Credit: Tracy Bryant, UF/IFAS
How do pesticides affect pollinators?
The effects of pesticides on pollinators can broadly be classified into lethal and sublethal effects. Lethal effects occur when the pesticide directly kills either adult foraging bees or developing brood. Lethal effects are typically measured by exposing adult honey bees to different concentrations of the pesticide both in contact exposure and oral exposure and determining the lethal dose or lethal concentration that kills 50% of individuals (i.e., the LD/LC 50). The lethal toxicity of a pesticide is sometimes examined with developing brood or with other pollinator species (e.g., bumble bees) and can vary significantly across life stages and species (Mussen et al. 2004; Wade et al. 2019).
In addition to lethal effects, pesticides can have a variety of sub-lethal effects on bees that include impaired learning and memory in adult foragers, weakened immune systems in adults and brood, and a reduction in reproduction, including fewer new queens or fewer total offspring. Pesticide exposure can also affect behaviors directly relevant for pollination, such as the overall foraging activity of individual bees or the attraction of bees to the crop plants (Morandin et al. 2005; Mommaerts et al. 2010; Wu et al. 2011; Gill et al. 2012; Tschoeke et al. 2019).
Pesticides can also interact with one another, especially when they are applied in tank mixes. Some pesticides are known to have synergistic effects, i.e., they enhance the toxicity of other pesticides applied simultaneously. This is especially true for fungicides; while many fungicides are not highly toxic by themselves, they have been found to increase the toxicity of insecticides when both are applied together (Pilling and Jepson 1993; Manning et al. 2017; Wade et al. 2019; Bigante et al. 2021). Fungicides have also been found to have significant sublethal effects on bees, including weakening bee immune systems. For these reasons, while many fungicides are not considered highly toxic to bees, care should be taken when applying them during bloom, especially when applying them simultaneously with insecticides.
Selecting Pesticides During Blueberry Bloom
Insecticides and fungicides that are commonly applied during blueberry bloom in Florida are listed below in alphabetical order of the active ingredient (note that this may not be an exhaustive list) along with their general toxicity to bees and aspects of residual activity or persistence in the environment (Tables 1 and 2). The general toxicity rating is based on the LC/LD 50 to honey bees measured through contact and/or oral exposure with practically non-toxic > 50 ug/bee; low toxicity < 50 and > 11 ug/bee; moderate toxicity < 11 and > 2 ug/bee; and high toxicity < 2 ug/bee (1 ug = 1/1,000,000 g and 1 g ~ 1/30 oz). Note that, counterintuitively, higher-toxicity products have a lower LC/LD 50, indicating that less active ingredient is needed to result in 50% mortality. A high LD/LC 50 conversely means that a large amount of active ingredient is needed to result in 50% mortality, and thus the product is less toxic. When selecting a product to apply during bloom, it is important to look not only at its toxicity but at whether it is systemic, whether it will persist in the environment (i.e. have persistent residual activity), and whether it may produce synergisms (interactions with other chemicals that may increase toxicity of one or more of the chemicals).
Table 1. Fungicides applied during blueberry bloom, their toxicity and persistence, and special considerations. For residual activity, the half-life listed refers to the amount of time it takes for pesticide residue quantities to be reduced by half.
Table 2. Insecticides applied during blueberry bloom, their toxicity, persistence, and special considerations. For residual activity, the half-life listed refers to the amount of time it takes for pesticide residue quantities to be reduced by half.
Tips for Limiting Pesticide Effects on Bees and Other Pollinators
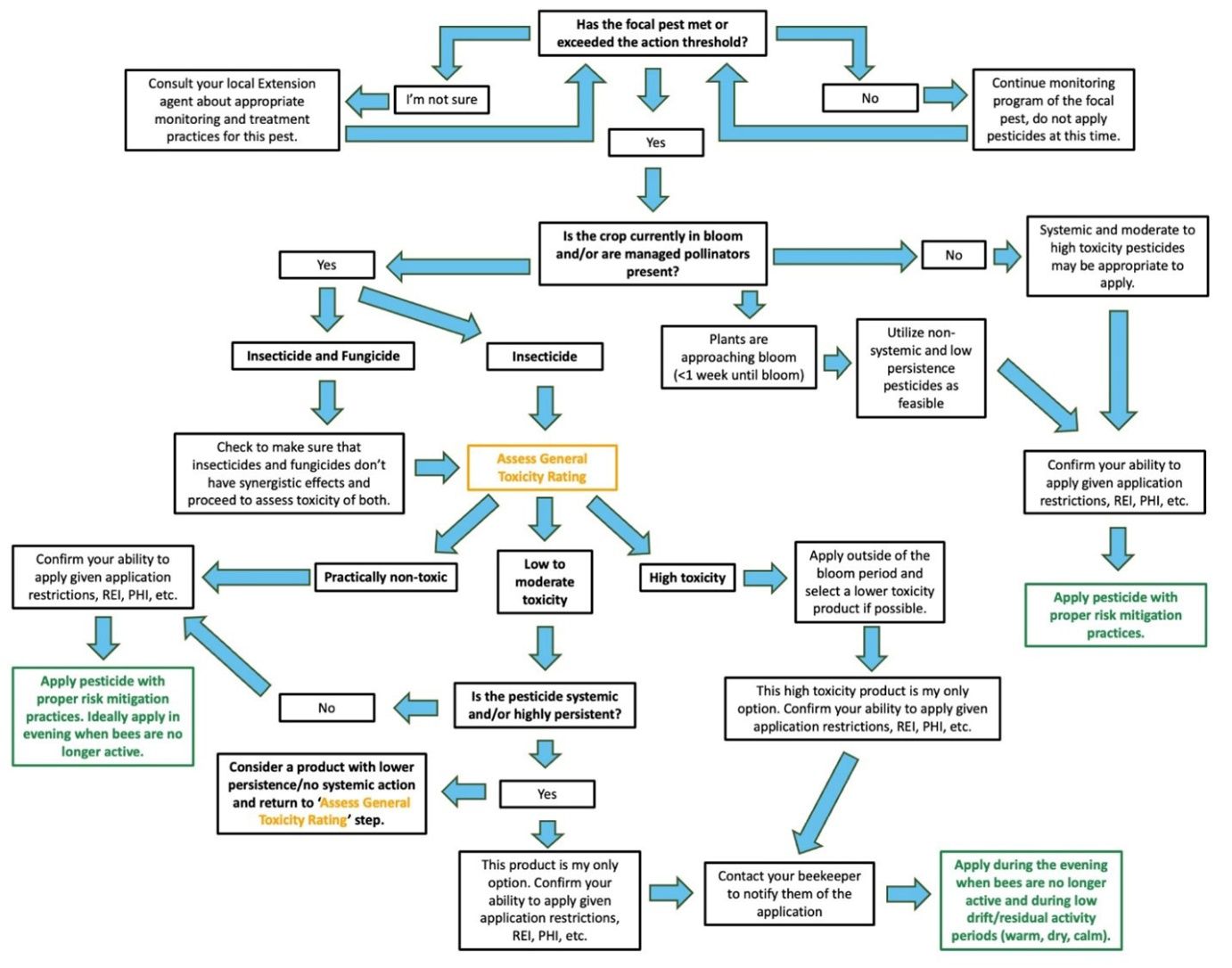
The following tips can help with decision making and reducing pollinator exposure to pesticides during the blueberry bloom period (Figure 2):
- Implement IPM strategies and other control methods to limit chemical sprays during bloom.
- Use existing action thresholds when possible.
- Follow label instructions. Many products with a high toxicity to bees specify that the product should not be applied in any mode of application during bloom and/or when bees are in the crop field.
- When possible, and especially during bloom, select a non-systemic insecticide with a short residual activity and no to low toxicity to bees.
- When possible, avoid using tank mixes of insecticides and fungicides during the bloom period to reduce synergistic effects.
- Apply pesticides, including fungicides and insecticides, in the evening to allow for the longest period of time to pass before bees forage. Cool temps and/or wet conditions may prolong residual activity.
- If using a pesticide with a moderate to high toxicity to bees during bloom, be sure to follow label instructions and also contact your beekeeper in advance so that they can consider moving or covering hives during application and for a period of time after application.
Credit: John Ternest and Rachel Mallinger, UF/IFAS
References
BASF. 2010. Safety Data Sheet Cabrio EG [Data Sheet]. BASF The Chemical Company.
Benjamin, F. E., and R. Winfree. 2014. “Lack of Pollinators Limits Fruit Production in Commercial Blueberry (Vaccinium corymbosum).” Environmental Entomology 43:1574–1583. https://doi.org/10.1603/EN13314
Besard, L., V. Mommaerts, G. Abdu-Alla, and G. Smagghe. 2011. “Lethal and Sublethal Side-Effect Assessment Supports a More Benign Profile of Spinetoram Compared with Spinosad in the Bumblebee Bombus terrestris.” Pest Management Science 67:541–547. https://doi.org/10.1002/ps.2093
Brigante, J., J. O. Costa, E. L. G. Espíndola, and M. A. Daam. 2021. “Acute Toxicity of the Insecticide Abamectin and the Fungicide Difenoconazole (Individually and in Mixture) to the Tropical Stingless Bee Melipona scutellaris.” Ecotoxicology 30:1872–1879. https://doi.org/10.1007/s10646-021-02458-7
Campbell, J. W., C. B. Kimmel, M. Bammer, C. Stanley-Stahr, J. C. Daniels, and J. D. Ellis. 2018. “Managed and Wild Bee Flower Visitors and Their Potential Contribution to Pollination Services of Low-Chill Highbush Blueberry (Vaccinium corymbosum L.; Ericales: Ericaceae).” Journal of Economic Entomology 111:2011–2016. https://doi.org/10.1093/jee/toy215
Danka, R. G., G. A. Lang, and C. L. Gupton. 1993. “Honey Bee (Hymenoptera: Apidae) Visits and Pollen Source Effects on Fruiting of ‘Gulfcoast’ Southern Highbush Blueberry.” Journal of Economic Entomology 86:131–136. https://doi.org/10.1093/jee/86.1.131
Federoff, N. E., J. Melendez, and F. Khan. 2001. EFED RED Chapter for Ziram [Memorandum]. United States Environmental Protection Agency, Washington, D.C.
Fisher, A., T. Cogley, C. Ozturk, G. DeGrandi-Hoffman, B. H. Smith, O. Kaftanoglu, J. H. Fewell, and J. F. Harrison. 2021. “The Active Ingredients of a Mitotoxic Fungicide Negatively Affect Pollen Consumption and Worker Survival in Laboratory-Reared Honey Bees (Apis mellifera).” Ecotoxicology and Environmental Safety 226:112841. https://doi.org/10.1016/j.ecoenv.2021.112841
Gad, S. C., and T. Pham. 2014. “Propiconazole.” In Encyclopedia of Toxicology (Third Edition), edited by P. Wexler, 1101–1104. Oxford: Academic Press. https://doi.org/10.1016/B978-0-12-386454-3.01203-3
Gajbhiye, V. T., S. Gupta, I. Mukherjee, S. B. Singh, N. Singh, P. Dureja, and Y. Kumar. 2011. “Persistence of Azoxystrobin in/on Grapes and Soil in Different Grapes Growing Areas of India.” Bulletin of Environmental Contamination and Toxicology 86:90–94. https://doi.org/10.1007/s00128-010-0170-2
Gill, R. J., O. Ramos-Rodriguez, and N. E. Raine. 2012. “Combined Pesticide Exposure Severely Affects Individual- and Colony-Level Traits in Bees.” Nature 491:105–108. https://doi.org/10.1038/nature11585
Goulson, D., 2013. “An Overview of the Environmental Risks Posed by Neonicotinoid Insecticides.” Journal of Applied Ecology 50 (4): 977–987. https://doi.org/10.1111/1365-2664.12111
Lewis, K. A., J. Tzilivakis, D. J. Warner, and A. Green. 2016. “An International Database for Pesticide Risk Assessments and Management.” Human and Ecological Risk Assessment: An International Journal 22:1050–1064. https://doi.org/10.1080/10807039.2015.1133242
Long, E. Y., and C. H. Krupke. 2016. “Non-Cultivated Plants Present a Season-Long Route of Pesticide Exposure for Honey Bees.” Nature Communications 7 (1): 11629. https://doi.org/10.1038/ncomms11629
Main, A. R., J. V. Headley, K. M. Peru, N. L. Michel, A. J. Cessna, and C. A. Morrissey. 2014. “Widespread Use and Frequent Detection of Neonicotinoid Insecticides in Wetlands of Canada's Prairie Pothole Region.” PLOS ONE 9 (3): 92821. https://doi.org/10.1371/journal.pone.0092821
Mallinger, R., J. J. Ternest, and S. M. Naranjo. 2021. “Blueberry Yields Increase with Bee Visitation Rates, but Bee Visitation Rates Are Not Consistently Predicted by Colony Stocking Densities.” Journal of Economic Entomology 114:1441–1451. https://doi.org/10.1093/jee/toab111
Manning, P., K. Ramanaidu, and G. C. Cutler. 2017. “Honey Bee Survival Is Affected by Interactions between Field-Relevant Rates of Fungicides and Insecticides Used in Apple and Blueberry Production.” FACETS 2:910–918. https://doi.org/10.1139/facets-2017-0025
Maus, C. 2008. “Ecotoxicological Profile of the Insecticide Spirotetramat.” Bayer CropSci. J. 61.
Mayes, M. A., G. D. Thompson, B. Husband, and M. M. Miles. 2003. “Spinosad Toxicity to Pollinators and Associated Risk.” Reviews of Environmental Contamination and Toxicology 179:37–71. https://doi.org/10.1007/0-387-21731-2_2
Miles, M., M. Mayes, and R. Dutton. 2002. “The Effects of Spinosad, a Naturally Derived Insect Control Agent, to the Honeybee (Apis melifera).” Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet 67:611–616.
Minnesota Department of Agriculture. 2014. Tolfenpyrad New Use Review [Fact Sheet]. St. Paul, MN, USA.
Mommaerts, V., S. Reynders, J. Boulet, L. Besard, G. Sterk, and G. Smagghe. 2010. “Risk Assessment for Side-Effects of Neonicotinoids against Bumblebees With and Without Impairing Foraging Behavior.” Ecotoxicology 19:207–215. https://doi.org/10.1007/s10646-009-0406-2
Morandin, L. A., M. L. Winston, M. T. Franklin, and V. A. Abbott. 2005. “Lethal and Sub-Lethal Effects of Spinosad on Bumble Bees (Bombus impatiens Cresson).” Pest Managment Science 61:619–626. https://doi.org/10.1002/ps.1058
Mussen, E. C., J. E. Lopez, and C. Y. S. Peng. 2004. “Effects of Selected Fungicides on Growth and Development of Larval Honey Bees, Apis mellifera L. (Hymenoptera: Apidae).” Environmental Entomology 33:1151–1154. https://doi.org/10.1603/0046-225X-33.5.1151
Nauen, R, P. Jeschke, R. Velten, M. E. Beck, U. Ebbinghaus-Kintscher, W. Thielert, K. Wölfel, M. Haas, K. Kunz, and G. Raupach. 2015. “Flupyradifurone: A Brief Profile of a New Butenolide Insecticide.” Pest Management Science 71:850–862. https://doi.org/10.1002/ps.3932
Olker, J. H., C. M. Elonen, A. Pilli, A. Anderson, B. Kinziger, S. Erickson, M. Skopinski, A. Pomplun, C. A. LaLone, C. L. Russom, and D. Hoff. 2022. “The ECOTOXicology Knowledgebase: A Curated Database of Ecologically Relevant Toxicity Tests to Support Environmental Research and Risk Assessment.” Environmental Toxicology and Chemistry 41:1520–1539. https://doi.org/10.1002/etc.5324
Pilling, E. D., and P. C. Jepson. 1993. “Synergism between EBI Fungicides and a Pyrethroid Insecticide in the Honeybee (Apis mellifera).” Pest Management Science 39:293–297. https://doi.org/10.1002/ps.2780390407
Rogers, S. R., D. R. Tarpy, and H. J. Burrack. 2014. “Bee Species Diversity Enhances Productivity and Stability in a Perennial Crop.” PLOS ONE 9:e97307. https://doi.org/10.1371/journal.pone.0097307
Stoner, K. A., and B. D. Eitzer. 2012. “Movement of Soil-Applied Imidacloprid and Thiamethoxam into Nectar and Pollen of Squash (Cucurbita pepo).” PLOS ONE 7:e39114. https://doi.org/10.1371/journal.pone.0039114
Tschoeke, P. H., E. E. Oliveira, M. S. Dalcin, M. C. A. C. Silveira-Tschoeke, R. A. Sarmento, and G. R. Santos. 2019. “Botanical and Synthetic Pesticides Alter the Flower Visitation Rates of Pollinator Bees in Neotropical Melon Fields.” Environmental Pollution 251:591–599. https://doi.org/10.1016/j.envpol.2019.04.133
United States Environmental Protection Agency, 1997. EPA Pesticide Fact Sheet Azoxystrobin [Fact Sheet]. US EPA, Washington D.C.
United States Environmental Protection Agency, 1998. EPA Pesticide Fact Sheet Mono- and d-potassium salts of phosphorous acid [Fact Sheet]. US EPA, Washington D.C.
United States Environmental Protection Agency, 1999. EPA R.E.D. Facts Captan [Fact Sheet]. US EPA, Washington D.C.
United States Environmental Protection Agency, 1999. EPA Pesticide Fact Sheet Fenhexamid [Fact Sheet]. US EPA, Washington D.C.
United States Environmental Protection Agency, 1999. EPA Reregistration Eligibility Decision Chlorothalonil [Archive Document]. US EPA, Washington D.C.
United States Environmental Protection Agency, 2001. EPA Pesticide Fact Sheet Fluazinam [Fact Sheet]. US EPA, Washington D.C.
United States Environmental Protection Agency, 2002. EPA Pesticide Fact Sheet Acetamiprid [Fact Sheet]. US EPA, Washington D.C.
United States Environmental Protection Agency, 2003. EPA Pesticide Fact Sheet Boscalid [Fact Sheet]. US EPA, Washington D.C.
United States Environmental Protection Agency, 2004. EPA R.E.D. Facts Ziram [Fact Sheet]. US EPA, Washington D.C.
United States Environmental Protection Agency, 2007. EPA Pesticide Fact Sheet Metconazole [Fact Sheet]. US EPA, Washington D.C.
Wade, A., C.-H. Lin, C. Kurkul, E. R. Regan, and R. M. Johnson. 2019. “Combined Toxicity of Insecticides and Fungicides Applied to California Almond Orchards to Honey Bee Larvae and Adults.” Insects 10:20. https://doi.org/10.3390/insects10010020
Wilhelmy, H. 2004. JAU 6476 a.i. acute effects on the honeybee Apis mellifera. [Laboratory Report]. Dr. U. Noach Laboratorium, Sarstedt, Germany.
Whitehorn, P. R., S. O’Connor, F. L. Wackers, and D. Goulson. 2012. “Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production.” Science 336:351–352. https://doi.org/10.1126/science.1215025
Wu, J. Y., C. M. Anelli, and W. S. Sheppard. 2011. “Sub-Lethal Effects of Pesticide Residues in Brood Comb on Worker Honey Bee (Apis mellifera) Development and Longevity.” PLoS ONE 6:e14720. https://doi.org/10.1371/journal.pone.0014720
Yang, E. C., Y. C. Chuang, Y. L. Chen, and L. H. Chang. 2008. “Abnormal Foraging Behavior Induced by Sublethal Dosage of Imidacloprid in the Honey Bee (Hymenoptera: Apidae).” Journal of Economic Entomology 101:1743–1748. https://doi.org/10.1603/0022-0493-101.6.1743