Introduction
Orchids are very popular as indoor house plants throughout the country and especially as outdoor plants in south Florida. Orchid foliage is tough, but developing flower buds and petals are tender. Orchid pests damage petals and destroy flowers, creating disappointment for homeowners and lost revenue for growers.
Unfortunately, several key insect and mite pests commonly infest orchids. The most frequent arthropod pests of concern are those with piercing-sucking mouthparts. In general, these pests cause orchids to become unthrifty and chlorotic, and damaged plants produce fewer flowers. Eventually, heavily infested plants die.
Insect and mite management requires awareness of the key arthropod pests. The most serious pests are small and easily overlooked. The orchid plant architecture and coarse rooting medium provide nooks and crannies where small insect pests can thrive undetected. Hence, monitoring pest activity requires frequent and close examination of your plants.
This publication is intended for orchid enthusiasts, whether homeowners or professional growers, to help picture-identify pests and symptoms and manage key orchid arthropod problems to produce healthier, better-blooming plants. It describes and suggests management strategies for mites and five key insect groups that cause the bulk of arthropod pest problems for orchid growers.

Credit: Doug Caldwell, UF/IFAS
Scale Insects
A Brief Biology
There are several groups of scale insects, but the two main groups of scale insect pests affecting orchids are armored scales and soft scales. Compared to soft scale species, armored scale species are small and flat, with adult females measuring about 1/16 inch long (1 to 2 mm long).
With soft scale species, by contrast, the adult females are larger, about the size of a plant bud in the 1/8-inch range (3 mm). They have a somewhat rounded profile. Soft scale species excrete “honeydew,” a rich, sugary liquid. A black fungal growth called sooty mold often colonizes the honeydew, sometimes in thick layers. Sooty mold is quite noticeable and can be a red flag of an active infestation.
Armored scale insects are unique in that their top covering (or test) is detachable and can be flipped off or removed (Figure 1). The soft scale species do not have a removable shield or top “armor.” The reproductive capacity of armored scales is lower than that of soft scale species and may range from 25 to 100 eggs per female. The soft scale group reproductive capacity ranges from about 400 to 3,000 eggs per female depending on the species. Typical scale life cycles begin with the egg stage and the first instar emerging from eggs produced under the female scale covering. The first instar, also referred to as the crawler stage, is the dispersal stage. They molt two or three times before they become mature. After the crawlers insert their piercing-sucking mouthparts into the plant tissues, armored scale species remain anchored in one location for the rest of their lives, but certain species of soft scale have mobility as nymphs and adults.
Boisduval scale
The Boisduval scale (Diaspis boisduvalii), an armored scale, is one of the most common and damaging orchid pests, as well as one of the most challenging to control (Johnson 2010; Gill 1997). Just one or a few scales can seriously debilitate plants and make them unsalable. This scale will kill orchids if it is not controlled promptly (Johnson 2010).
Description
The female Boisduval scale covering is circular, transparent, and colorless to whitish yellow. It measures about 1.2 to 2.25 mm in diameter (Figure 1). The males, on the other hand, are white and elongate with three longitudinal ridges, and so dissimilar to the females that they look like a different species of scale. They are about 1 mm long (Figure 1).

Credit: Doug L. Caldwell, UF/IFAS
Biology
The Boisduval scale only needs about 50 days to go through all the life stages to complete a generation. Females produce up to 200 eggs in their life span (Gill 1997). The males produce white, waxy strands and tend to gather in aggregations (Figures 1 and 2 top right), which are sometimes mistaken for infestations of mealybugs or wax-producing whiteflies. There are many overlapping generations per year, which means constant, year-round scouting is important for early pest detection.
Damage Symptoms
The long, threadlike, piercing-sucking mouthparts of Boisduval scale, called stylets, pierce and damage leaf cells causing chlorotic depressions (Figure 2) and plant decline. A single scale can produce broad zones of cell death, resulting in chlorosis and necrosis (Johnson 2010).
Scouting Tips and Management
Boisduval scale favors Cattleya and Cymbidium orchids (Gill 1997), Laeliine and Oncidiine orchids, and at least 34 other orchid genera (Johnson 2010). Examine all sides of the leaves for yellowing, discolored areas (Figure 2). Check the leaf axils and the base of the pseudobulbs closely, and carefully remove papery sheaths to expose hidden pests (Figure 2 bottom photo).
It is important to identify which pest(s) are attacking your orchids to best select a chemical that is effective on that particular pest species. For example, certain systemic chemicals that are effective on mealybugs and whiteflies (such as imidacloprid), are not effective on armored scales. Armored scales feed on the contents of mesophyll cells and do not feed from the vascular pipelines where certain systemic insecticides are translocated.
Boisduval scale can easily be misidentified as mealybugs and vice versa. Try using a small water-color paintbrush to gently probe the white insects. If they brush off when probed, it is probably a mealybug or whitefly infestation. Scale insects are not easy to dislodge. The armored scales are attached to the plant by their waxy excretions and sucking mouthparts, like a button sewn to a shirt. Another scouting reminder is that armored scales do not excrete “honeydew,” the sticky, sugar-rich excretion of soft scales, mealybugs and certain whiteflies that attracts ants.
One reason this scale can be a persistent pest is that it feeds on many different plants, such as: palm species, bromeliads, banana, and other outdoor landscape plants in south Florida (Espinosa et al. 2020). Nearby outdoor plants or weeds can harbor infestations that can readily infest orchids and suddenly attack what were considered “clean” plants.
Other armored scale species that may also attack orchids include proteus scale, Parlatoria proteus and Florida red scale, Chrysomphalus aonidum (Watson 2008).

Credit: Doug L. Caldwell, UF/IFAS
What to do? See the management chart (Table 1) at the end of this publication.
Brown soft scale
Description
The adult female scales (Figure 3) range from 3 to 5 mm long and produce 80 to 250 eggs each. Adult female brown soft scale lacks distinguishing features. Its color and shape are variable. The color of adult scales ranges from pale yellow-brown to greenish with variable patterns of irregular brown spots. The shape is slightly convex but may be irregular to fit into tight crevices of the orchid architecture.
Biology
There may be three to five generations per year. Each female produces 80 to 250 eggs. The eggs remain protected underneath the body of the female, as do the first instar nymphs (also called crawlers), which hatch from beneath the female. Males resemble tiny winged gnats and are vastly less common than females in this species (Kessing and Mau 1992).
Damage Symptoms
The piercing-sucking damage causes yellowing and loss of plant vigor. Secondary or indirect damage may occur as a result of the sooty mold that often grows upon the copious honeydew excreted by this scale species. Sooty mold interferes with photosynthesis, and it is unsightly, which creates an aesthetic concern and decreases the marketability of plants. Unlike Boisduval scale, which causes noticeable damage with even a single insect present, individual brown soft scale insects cause only slight damage.
Scouting Tips and Management
Because brown soft scales are nearly transparent in the younger stages (Figure 3) and the adults resemble plant buds, they are likely to be overlooked until sooty mold or ants appear. Monitor for the shiny honeydew and subsequent sooty mold and ants moving around on the plants. Brown soft scale is highly polyphagous, which means that it feeds on a very broad range of plants. It will thrive on plants from 417 genera, including ferns, palms, citrus, loquat, and many ornamental species (Garcia et al. 2016).

Credit: Top, Lyle J. Buss, UF/IFAS; Bottom, Doug L. Caldwell, UF/IFAS
Another soft scale species that attacks orchids is the hemispherical scale, Saissetia coffeae.
What to do? See the management chart (Table 1) at the end of this publication.
Mealybugs
Among orchid pests, mealybugs are notorious for being very difficult to manage, let alone control. The difficulty in controlling them is in part due to the protective white, “mealy,” waxy dusting of their exoskeleton, from which their common name is derived. Mealybugs that attack orchids include the longtailed mealybug, Pseudococcus longispinus (Figure 4, top); Pseudococcus dendrobiorum (Figure 4, bottom), a 2009 exotic arrival; the pineapple mealybug, Dysmicoccus brevipes; the Jack Beardsley mealybug, P. jackbeardsleyi; and the obscure mealybug, P. viburni (Johnson 2009).

Credit: Lyle J. Buss, UF/IFAS
Description
Mealybug species are identified superficially by the length of their marginal spikes or filaments and their body color. The longtailed mealybug is not unique in having the long, tail-like, waxy filaments; Jack Beardsley and obscure mealybugs also have long tail filaments. The adult female longtailed mealybug is about 3 to just under 4 mm long, exclusive of the tail filaments, which can be as long as the body or even longer.
Biology
Mealybugs have piercing-sucking mouthparts and are much more ambulatory than most scale insects. When disturbed, they will readily crawl away. Most mealybugs go through three immature (nymphal instars) developmental stages, which are miniature versions of the 2- to 5-mm-long adult females. The tiny, two-winged males are rarely seen; they are not abundant and only live a few days (Tenbrink and Hara 1993). The longtailed mealybug has two to four overlapping generations per year. Upon maturing, each female may produce 100 to 200 offspring in a few weeks. The first instar nymphs (crawlers) of the longtailed mealybug emerge from the unseen egg almost immediately, appearing to skip the egg stage. With many other species of mealybugs, eggs are deposited beside the female in white, waxy, material called an ovisac.
Damage Symptoms
Mealybug feeding weakens the plant, causing a decline in overall vigor and loss of leaves, buds, and flowers. Foliage may be spotted, curled, or wilted. Mealybugs produce their waste in liquid form referred to as “honeydew.” This excretion is a sugary substrate for black fungal growth often called sooty mold. When it accumulates in thick layers, sooty mold can hinder photosynthesis and decrease marketability of plants.
Scouting Tips and Management
Mealybugs are both troublesome and practically ubiquitous. Various species may feed on many types of greenhouse plants whether vegetable plants in the garden or houseplants.
Mealybugs are difficult to manage. They often hide in the nooks and crannies of a plant’s root system, under stem sheaths, and in the planting media. The immature stages readily move around. They become exposed when populations reach high densities and have no choice but to relocate into the upper plant canopy.
Bottom (2021) reports that mealybugs are troublesome on Paphiopedilum and Phalaenopsis species and also attack tender new Cattleya growth. Check for sand and debris accumulations piled at the soil line around main stems. Ants will protect their honeydew supply by covering mealybugs to protect them from the elements and to keep other insects from attacking them (Gutting et al. 2015). Ants may also move mealybugs and create new infestations on nearby plants. Mealybugs are fairly mobile, themselves. They will move off plants and relocate to bench crevices and other inanimate objects such as pots and trays (Johnson 2009). Carefully examine newly emerging leaves and sheaths for young mealybugs. Mealybugs have multiple generations, so one needs to be on guard every week, all year to prevent new outbreaks.
What to do? See the management chart (Table 1) at the end of this publication.
Thrips
(A point of grammar: the word “thrips” is always spelled with an “s,” whether it refers to one thrips or 10 thrips.) Thrips can easily be overlooked because they have tiny developmental stages and cryptic habits, and because they develop rapidly and move fast . There are at least six species of thrips that have been reported as orchid pests (Hamon 2008).
The common name orchid thrips is associated with two species in the same genus: Chaetanophothrips orchidii and C. signapennis. Other pest species include the red-banded thrips, Selenothrips rubrocinctus; the Florida flower thrips, Frankliniella bispinosa; the western flower thrips, Frankliniella occidentalis; the greenhouse thrips, Heliothrips haemorrhoidalis; and the vanda thrips, Dicromothrips corbetti.
Description
Adult thrips (Figure 5 bottom left) of most species are spindle-shaped, narrow-bodied, black to dark brown (except C. orchidii and F. spinosa, which are pale yellow) and usually under 2 mm long. The immature stages resemble the adult and are especially difficult to see because they lack color and can be less than 0.5 mm long.

Credit: Doug L. Caldwell, UF/IFAS
Biology
Thrips have the following developmental stages: egg; two immature, mobile, incremental feeding stages that are a small version of the adult; two unique, non-feeding, inactive instars (pseudopupae); and adult. Many species leave the plant and go through a transformation to the adult stage in the soil or substrate. Thrips have crude wings comprised of basically a strap with scraggly hairs. They are poor fliers but drift readily with air currents.
Eggs are inserted into plant tissue. The greenhouse thrips produce 60 eggs per female. First instar thrips require about 15 days to emerge from the eggs for this species (de Souza et al. 2022). Most species complete their life cycle in about 21 days in Florida (Funderburk et al. 2007). There are overlapping generations all year.
Damage Symptoms
Thrips have unique, rasping-sucking mouthparts that scar plant tissue. They won’t necessarily kill plants, but they can ruin the blossoms that make people treasure orchids by distorting or killing the developing flower buds. Thrips can subtly distort and scar (decolorize) petals (Figure 5)—thrips damage can be confused with virus color-break symptoms—or they can cause more extreme, complete flower kill, destroying the developing flower stalk (raceme) and giving the flower buds a scorched appearance (Figure 6). They can also feed on the undersides of leaves, especially on thin-leaf orchids. Feeding may start as a small chlorotic spot, enlarge to a bull’s-eye, and finally expand to create a silvery, stippled appearance over a large area of the leaf (Bottom 2017c).

Credit: Doug L. Caldwell, UF/IFAS
Thrips have been reported to vector (spread) viruses in some orchids. Hu et al. (1992) reported tomato spotted virus disease was detected in Oncidium orchid nurseries in Hawaii, and the western flower thrips was confirmed to be the vector.
Scouting Tips and Management
Use blue or yellow sticky cards to monitor flying thrips (Figure 12). Mites cause stippling or bronzing of the foliage that looks similar to thrips damage, but some thrips leave tell-tale clues of dark-colored, shiny fecal spots. Mites do not leave these spots. Horticultural oil or soap sprays will need repeating because some thrips will be missed when they relocate to the substrate or soil to develop into adults.
One peculiar symptom on vanda roots is a uniform depression that encircles the roots and gives them a segmented appearance (Figure 7). Many people (Motes 2021) attribute this segmentation to thrips damage, but the symmetry of the segments argues against this explanation. The segmenting does not appear to harm plants and may, in fact, be natural growth rings or some response to an abiotic factor.

Credit: Doug L. Caldwell, UF/IFAS
What to do? See the management chart (Table 1) at the end of this publication.
Whiteflies
Whiteflies are another piercing-sucking insect whose populations can quickly build up into large numbers in a short time. Whiteflies aren’t a frequent a pest on orchids as some of the previously discussed pests are, but one should nevertheless be on the look-out for them. In south Florida, new insects arrive through various shipping ports such as Miami and Tampa, so it is important to be alert for the unexpected as well as the known species of pests.
Dr. Erin Powell (Florida Department of Agricultural Services) reports (personal communication May 13, 2022) that the USDA’s diagnostic clinic rarely sees whiteflies on orchid samples. Since 1980, they only have six records in their database. These were three whitefly species:
- the spiraling whitefly, Aleurodicus dispersus on Phaius sp. and two undetermined Orchidaceae
- the rugose spiraling whitefly, Aleurodicus rugioperculatus on Phaius sp. and on Zeuxine strateumatica
- the giant whitefly, Aleurodicus dugesii on Calanthe sp.
Description
The photo on the top in Figure 8 shows the cottony egg trails and adults of Aleurodicus dispersus on the bell orchid, Grammatophyllum scriptum, and the photo on the bottom shows the decorative nature of immature A. dispersus whitefly stages. Immature stages of other whitefly species do not have as much body wax and may resemble immature stages of soft scale insects. These three whitefly species are about 3.5 mm long, which is about three times larger than most whitefly species.
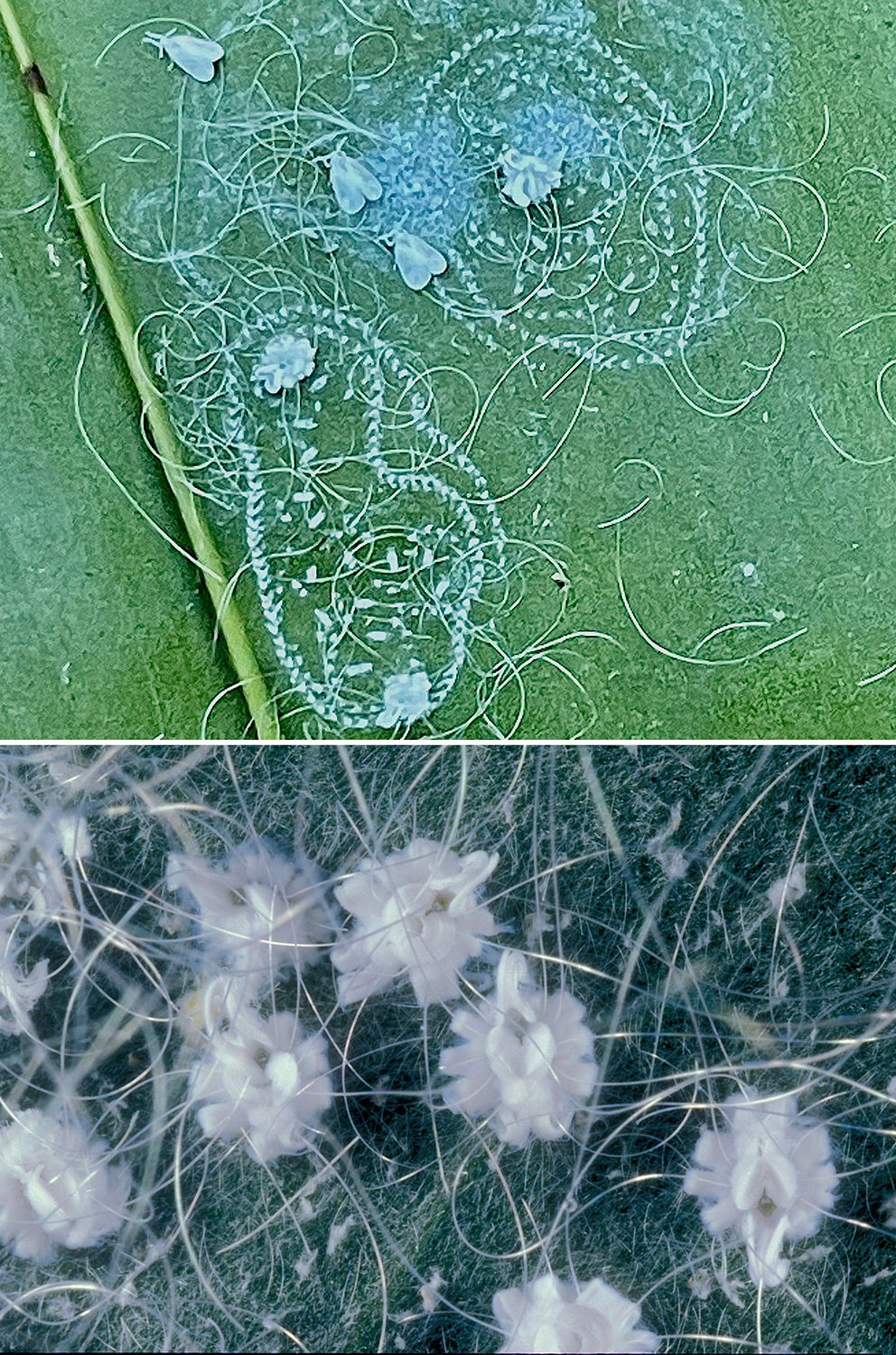
Credit: Top, Doug L. Caldwell, UF/IFAS; bottom, Lyle J. Buss, UF/IFAS
Biology
Whiteflies are not even closely related to true flies. Whiteflies have a different metamorphosis. Their developmental stages are: egg; crawler (first instar nymph); two flattened, non-mobile feeding, nymphal stages; a “resting,” non-feeding stage called the pseudopupa; and the winged adult. It takes the rugose spiraling whitefly about 30 days (Kumar et al. 2013) to go through all these stages.
Damage Symptoms
Symptoms can be subtle at first, but as the whiteflies feed from the sap stream, a general malaise, yellowing, and stunting may occur.
Scouting Tips and Management
Fortunately, these species are large and easy to spot because of all the white waxy material they produce. The presence of sooty mold and ants and swarms of small white insects that appear when foliage is disturbed and resemble a snowstorm globe when it is turned upside down are also common indications of a whitefly infestation. Check the undersides of leaves, petals, and buds. Whiteflies can be rubbed off and smashed, or they may be treated with a pesticide listed in Table 1.
What to do? See the management chart (Table 1) at the end of this publication.
Mites
Orchids may be attacked by two main mite groups: spider mites (Tetranychidae) and flat mites (Tenuipalpidae). Perhaps the most common among these is the ubiquitous two-spotted spider mite, Tetranychus uriticae (Figure 9, top). In addition, at least four flat mite species attack orchids. These are sometimes called “false spider mites” because they do not produce the fine webbing characteristic of true spider mites (Figure 9, bottom).

Credit: Top, James L. Castner, UF/IFAS; bottom, Doug L. Caldwell, UF/IFAS
The two flat mite species that are the most damaging are Tenuipalpus pacificus, the phalaenopsis mite (Figures 10 and 11), which is sometimes called the omnivorous mite; and Brevipalpus californicus, the orchid mite (personal communication Dr. Alexandra Revynthi, UF/IFAS Tropical Research and Education Center, Homestead. February 9, 2023). Other damaging flat mites are Tenuipalpus orchidarum and the oncidium mite, Brevipalpus oncidii.

Credit: D. Shetlar
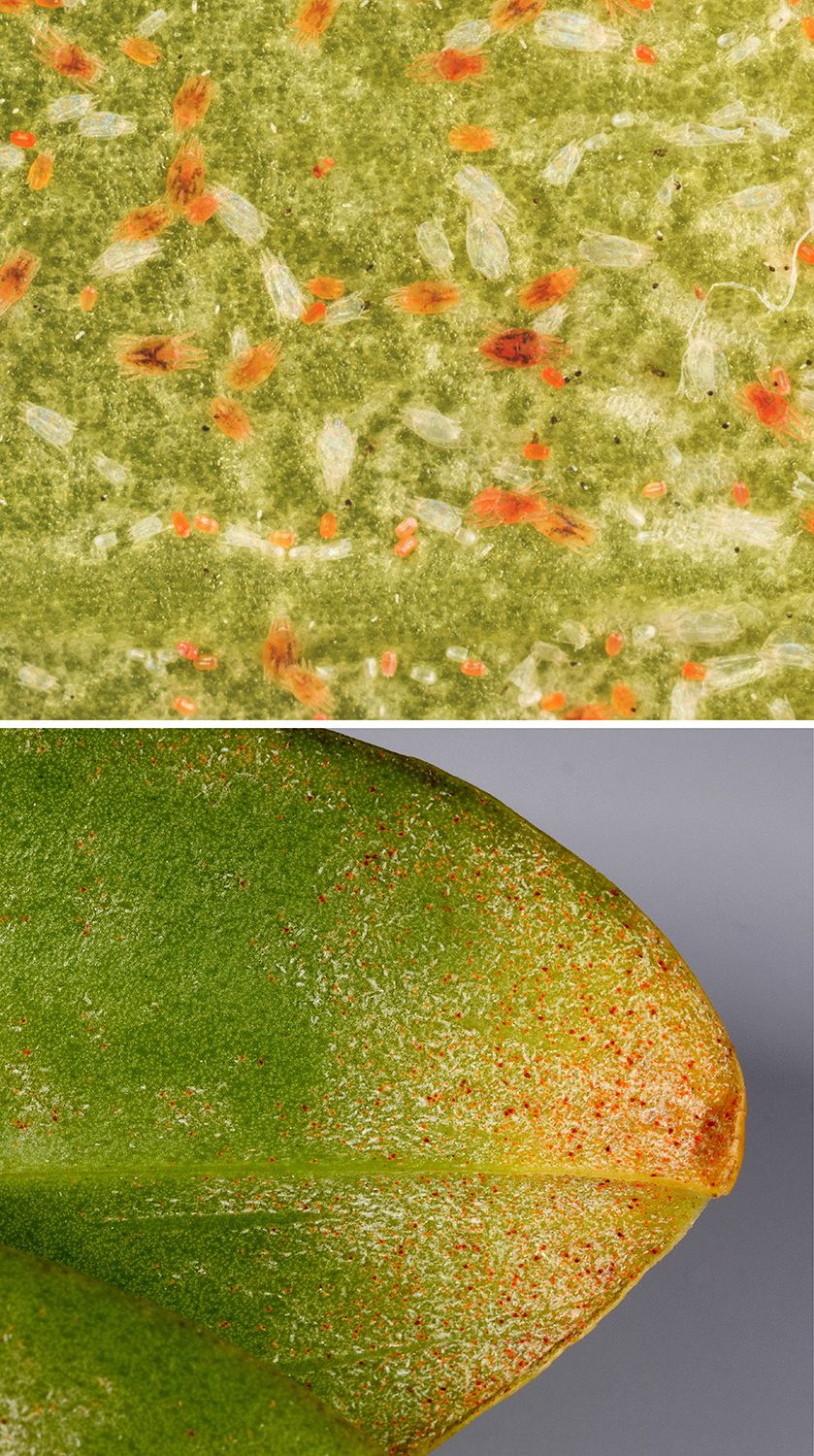
Credit: Lyle J. Buss, UF/IFAS
Description
Johnson reported (2008) that flat mites move very slowly and are smaller (0.3 mm long) than two-spotted spider mites (0.5 mm long). Many mite species have six legs as immatures and eight legs as adults. Mites have two body regions: a cephalothorax and an abdomen, whereas insects have 3 distinct body regions: head, thorax and abdomen and usually adult insects have six legs.
Grammatophylum, dendrobiums, and catasetum orchids are reported to be prone to mite infestations (Bottom 2021), but the mite species that affect them have not been identified. Orchids with thin leaves or soft leaves tend to suffer more mite damage than do thick-leafed species. Outdoors, mites tend to be more damaging when environmental humidity is low, for instance, in south Florida during the relatively dry, cool months of November through April.
Biology
Two-spotted spider mites (Figure 9) tend to begin feeding on the undersides of leaves. These mites can go through a generation from egg to adult rapidly in two to three weeks, or, under optimum conditions, in as little as five days (Johnson 2008). In quiet, sheltered areas and during rain-free periods, spider mites produce webbing (Figure 9, bottom). Flat mites, too, prefer to feed on the undersides of leaves. Flat mites require from three to eight weeks to produce a generation (Johnson 2008). All of these mite species have multiple generations per year, and they can float on air currents from many different nearby hosts, all of which makes them quite difficult to manage.
Mites in the genus Brevipalpus are vectors of plant viruses. B. californicus mites transmit the orchid fleck virus to orchids and certain monocot groundcover species (Fife et al. 2021).
Damage Symptoms
One has to be acutely observant to find these tiny arthropods. Mites have a pair of miniscule, knife-like lacerating mandibles they use to mash the mesophyll cells, and then they suck up the leaking fluids and chlorophyll with their gnathosoma, which is essentially a large upper lip. Spider mite feeding sucks the fluids from cells, leaving stippled or whitish areas on foliage that progress to tan or bronze patches. Flat mite feeding creates more of a pock-marked appearance (Figure 11, top) that becomes silvery and then rusty black. Look for their shed, white exoskeletons, which stand out against the rusty black color of damaged leaves. Mites can disfigure or even kill plants if ignored.
Scouting Tips and Management
Webbing (Figure 9, bottom) and stippling or yellow speckles can indicate spider mite activity. The much smaller flat mite does not produce webbing, and its feeding damage has more of a pock-marked, stippled appearance. Both types of mites prefer to feed on the undersides of the leaves.
What to do? See the management chart (Table 1) at the end of this publication.
Other Orchid Pests
In addition to the pests described above, orchid growers may occasionally encounter aphids; lubber grasshoppers, Romalea microptera; blossom midge, Contarinia maculipennis; caterpillars; the dark flower beetles, Euphoria sepulcralis; and snails and slugs. Information on other pests may be found in several of the listed references including Bottom 2017 b and Watson 2008.
Management Overview for Orchid Insect and Mite Pests of South Florida
Pesticides are probably overused in orchid culture. A survey of orchid growers in Hawaii (Hollingsworth et al. 2000; Table 3) showed that, of the 24 orchid growers who responded to the survey, the 16 who applied pesticides on a calendar basis used an average of 46 pesticide applications per year; but the eight growers who scouted to determine pest population threats made 25 applications, a 54% reduction in insecticide use. Thrips were listed as key pests for cut orchid growers in this survey, but the species were not provided.
For homeowners, orchid arthropod management decisions should be based on frequent, perhaps weekly, scouting. When pests are found, spot-treat to help protect orchid flower production. Avoid outbreaks when acquiring new plants by quarantining them for two to four weeks and applying a pesticide treatment as insurance.
Learn to spot the orchid pests described in this publication and check nearby plants in landscapes and greenhouses for them. The orchid pests discussed above are particularly adaptable and can survive on many different plant species. Eliminate neighboring infestations on alternative hosts immediately. Scout all nearby plants, even weeds, and remove the plant or treat the pest at the first sign of a problem to avoid an infestation.
Growers tend to use regular, preventive pesticide spray schedules, rather than scouting, to protect their investment. Integrated pest management (IPM) encourages observing the pests before treating, but orchid pests are very small and easy to miss, and if they are overlooked even for a few days, they can cause damage severe enough to kill flowers and destroy plant marketability. This is especially true of thrips. Preventive spraying therefore often makes sense particularly if there is a history of infestation or very susceptible plants. Sometimes severely infested plants should be discarded to protect the remaining orchids.
If bees and other pollinators visit your orchids, then avoid systemic insecticides including acephate and the neonicotinoids. These insecticides may transfer into the nectar or pollen and injure pollinators. Instead, consider using horticultural mineral oil (Borden et al. 2018) or horticultural soap sprays (Borden and Dale 2019). These oil and soap spray results are very short-term and only affect what you spray; that is, “what you hit is what you get,” is a rule when using oils and soaps. Soap and oil sprays do not offer significant residual benefits. During sunny or hot weather, insecticidal soaps may damage foliage and flower buds. Be careful to follow the label mixing doses and temperature precautions to avoid plant injury when using these and any insecticide products. Remember, the label is the law. Label instructions must be followed exactly to protect your plants, yourself, and the environment.

Credit: Toncho Bogoev, Aris Horticulture. Alva, Florida
Touch-up sprays with horticultural mineral oil and other short residual pesticides (Borden and Dale 2019; Borden et al. 2018) for mites, and the other pests mentioned, should be repeated because surviving pests will emerge after treatment from sheltered areas in the nooks and crannies of plants and in the potting media. Repeat roughly three times every five to seven days until the pests cease to emerge.
Try using yellow or white sticky cards to monitor thrips, whiteflies, fungus gnats, and other flying pests. Locate them where workers and lizards are less likely to be snared (Figure 12).
Orthene dependence could lead to pest resistance
From discussions with growers and orchid hobbyists, it seems that the odiferous acephate (sold under the brand name Orthene® and others) has been used as the favored cure-all for these pests for many years. This frequent reliance on a single active ingredient may lead to the development of insecticide resistance with target species and poorer control of those species. To minimize the chance of pesticide resistance, pesticide management experts universally recommend rotating to a different IRAC mode of action (see IRAC in references). Common orchid insecticides and their IRAC class are listed by Sue Bottom (2017a) and in Table 1.
Various combinations of imidacloprid + clothianidin and dinotefuran and other neonicotinoids should be worthy substitutes for acephate. The neonicotinoids are foliar systemics and many are also absorbed by the roots and translocated throughout the entire plant. The newer products cost more, but the increased cost is offset by their longer residual efficacy. Growers could enjoy months rather than seven to ten days of control or suppression, and all without the unpleasant, lingering, sulfur smell of acephate. More information is needed on the duration of neonicotinoid efficacy with different orchid species and their cultural setups, especially with different soilless potting media, such as bark or lava stone. Most data and experiences with neonicotinoids have been with trees and shrubs in various landscape soils. Again, neonicotinoids and other systemic insecticides will injure bees and other pollinators.
For more treatment alternatives, see the lists posted at the St. Augustine Orchid Society by Sue Bottom. Stay tuned for UF/IFAS Extension recommendations for new products entering the marketplace.
Summary
This publication provides macro-pictures and information to help identify and manage the most common and damaging insect and mite pests of orchids in south Florida. This information was acquired from interviews with local nursery staff in the Ft. Myers and Naples, Florida, area, as well as with local orchid club members. These orchid pests are tiny, so frequent plant examination is necessary to prevent serious outbreaks and damage. Keep current on new pests and management strategies through your local UF/IFAS Extension horticulture educators. Excellent information is also found at the American Orchid Society (https://www.aos.org/) and the St. Augustine Orchid Society (https://staugorchidsociety.org/culturepests-pests.htm) websites.
Acknowledgments
To the unnamed manuscript reviewers, thank you for your time and wisdom; and much gratitude to Lyle J. Buss (UF/IFAS Entomology and Nematology Department) and Dr. John Finer (Ohio State University emeritus) for providing ideas and edits early in the development of this paper; Drs. Erin C. Powell, Susan Halbert, Felipe Soto-Adames from the Florida Department of Agriculture and Consumer Services, Division of Plant Industry, for orchid insect pest identification records and frequency data. Thank you to Twyla Leigh for project creation and helping get it done, four years after I retired.
Selected References
Borden, M. A., and A. G. Dale. 2019. “Managing Plant Pests with Soaps: ENY 344/IN1248, 6/2019.” DIS 2019 (4). Gainesville, Florida. https://doi.org/10.32473/edis-in1248-2019
Borden, M. A., E. A. Buss, S. G. Park Brown, and A. G. Dale. 2018. “Natural Products for Managing Landscape and Garden Pests in Florida: ENY-350/IN197 Major, 9/2018.” EDIS 2018 (5). Gainesville, Florida. https://doi.org/10.32473/edis-in197-2018
Bottom, S. 2017a. “Orchid Pests and Diseases—Orchid Pests. Insecticides and Miticides for the Treatment of Orchid Pests.” St. Augustine Orchid Society. https://staugorchidsociety.org/PDF/PesticidesforOrchidPestsbySueBottom.pdf
Bottom, S. 2017b. “Orchid Pests and Diseases, Diagnosis, Treatment and Prevention.” St. Augustine Orchid Soc. 57 pp. https://staugorchidsociety.org/PDF/OrchidPestsandDiseasesbySueBottom.pdf
Bottom, S. 2017c. “Thrips on Orchids.” https://staugorchidsociety.org/PDF/ThripsonOrchidsbySueBottom.pdf
Bottom, S. 2020. “The Usual Suspects—Common Orchid Pests.” St. Augustine Orchid Society. https://www.staugorchidsociety.org/PDF/UsualSuspects-Common%20Orchid%20ProblemsbySueBottom.pdf
de Souza, M. T., M. T. de Souza, and M. A. C. Zawadneak. 2022. “Biology and Life Table Parameters of the Heliothrips haemorrhoidalison on Strawberries.” Phytoparasitica. 50: 35–41. https:/doi.org/10.1007/s12600-021-00943-7
Espinosa, A., H. Bowman, A. Hodges, and G. Hodges. 2020. “Boisduval Scale, Diaspis Boisduvalii Signoret (Insecta: Hemiptera: Diaspididae): EENY-467/IN838, 11/2009.” EDIS 2009 (10). Gainesville, Florida. https://doi.org/10.32473/edis-in838-2009
Fife, A., D. Carrillo, G. Knox, F. Iriarte, K. Dey, A. Roy, R. Ochoa, G. Bauchan, M. Paret, and X. Martini. 2021. “Brevipalpus-Transmitted Orchid Fleck Virus Infecting Three New Ornamental Hosts in Florida.” Journal of Integrated Pest Management. 12 (43): 1–6. 10.1093/jipm/pmab035
Funderburk, J., S. Diffie, J. Sharma, A. Hodges, and L. Osborne. 2007. “Thrips of Ornamentals in the Southeastern US: ENY-845/IN754, 12/2007.” EDIS 2008 (1). Gainesville, Florida. https://doi.org/10.32473/edis-in754-2007
Garcia, M. M., B. D. Denno, D. R. Miller, G. L. Miller, Y. Ben-Dov, and N. B. Hardy. 2016. “ScaleNet: A Literature-Based Model of Scale Insect Biology and Systematics.” Database: The Journal of Biological Databases and Curation. https://doi.org/10.1093/database/bav118
Gill, R. J. 1997. “The Scale Insects of California. Part 3. The Armored Scales (Homoptera: Coccoidea: Coccidae).” Technical Series in Agricultural Biosystematics and Plant Pathology No.3. California Department of Food and Agriculture, Sacramento, Calif., USA. 307 pp.
Gutting, A., J. A. Zettler, L. W. Zettler, and L. W. Richardson. 2015. “An Update on Mealybugs and Scale Insects (Hemiptera) on Native Epiphytic Orchids in South Florida, Including a New Record for Pseudococcus microcirculus (Pseudococcidae).” Florida Entomologist 98 (2): 401–404. https://doi.org/10.1653/024.098.0201
Hara, A. H., and R. Y. Niino-DuPonte. 2002. “Contarinia maculipennis. Blossom Midge.” http://www.extento.hawaii.edu/kbase/crop/Type/bloss_midgei.htm
Hollingsworth, R. G., A. H. Hara, and K. T. Sewake. 2000. “Pesticide Use and Grower Perceptions of Pest Problems on Ornamental Crops in Hawaii.” Journal of Extension 38 (1): 1RIB1. 8 pp. https://archives.joe.org/joe/2000february/rb1.php
Hollingsworth, R. G., K. T. Sewake, and J. W. Armstrong. 2002. “Scouting Methods for Detection of Thrips (Thysanoptera: Thripidae) on Dendrobium Orchids in Hawaii.” Environmental Entomology 31 (3): 523–532. https://doi.org/10.1603/0046-225X-31.3.523
Hu, J. S., M. Wang, S. Ferreira, and D. Ogata. 1992. “Tomato Spotted Wilt Virus on Oncidium orchids in Hawaii.” Plant Disease 76:426. https://doi.org/10.1094/PD-76-0426D
IRAC Mode of Action Classification, Version 10.5. 2023. https://irac-online.org/documents/moa-classification./?ext=pdf pp 7-16.
Johnson, P. J. 2008. “Mites on Cultivated Orchids.” St. Augustine Orchid Society. https://staugorchidsociety.org/PDF/Johnson-Mites.pdf. 7 pp.
Johnson, P. J. 2009. “Mealybugs on Orchids.” St. Augustine Orchid Society. https://staugorchidsociety.org/PDF/Johnson-Mealybugs.pdf. 9 pp.
Johnson, P. J. 2010. Boisduval Scale on Orchids. Orchid Digest 74(3): 170–177.
Kessing, J. L. M, and R. F. L. Mau. 1992. “Coccus hesperidum (Linnaeus).” Crop Knowledge Master. http://www.extento.hawaii.edu/kbase/crop/Type/c_hesper.htm .
Kumar, V., G. Kakkar, C. L. Palmer, C. L. McKenzie, and L. S. Osborne. 2016. “Thrips Management Program for Horticultural Crops: ENY-987/IN1145, 9/2016”. EDIS 2016 (9). Gainesville, Florida. https://doi.org/10.32473/edis-in1145-2016
Kumar V., McKenzie C.L., Mannion C., Stocks I., Smith T., Osborne L.S. 2013. Rugose spiraling whitefly, Aleurodicus rugioperculatus Martin (Hemiptera: Aleyrodidae). EENY578. University of Florida, IFAS Extension, http://entnemdept.ufl.edu/creatures/orn/Aleurodicus_rugioperculatus.html
Motes, M. 2021. Florida Orchid Growing — Month by Month. 205 pp. (esp. 172–173). Redland Press, Redland Florida.
Tenbrink, V. L., and A. H. Hara. 1993. “Pseudococcus longispinus (Targioni-Tozzetti) Longtailed Mealybug.” Crop Knowledge Master. http://www.extento.hawaii.edu/kbase/crop/Type/p_longis.htm.
Watson, J. B., editor. 2008. Orchid Pests and Diseases. Ch. 8. Orchid pests by A. B. Hamon. 124 pp. (especially pp. 36–50) American Orchid Society. Delray Beach, FL.
Table 1. Some products listed for management of key orchid arthropod pests in south Florida. Contact: Insect Specialist Dr. Adam G. Dale (agdale@ufl.edu).