The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The nematode Heterodera glycines, known as soybean cyst nematode (SCN) (Figure 1), is the most economically damaging pest of soybean crops in the United States and around the world (Hunt, 2008; Yu, 2011; Zhou et. al., 2017; The SCN Coalition, 2023). Members of the Heteroderidae family belong in the order Rhabditida; most are plant parasitic while some are insect parasites. They are obligate parasites that attack different species of crops and weeds. The word heteros in Greek means ‘other’, deras ‘skin’ (Siddiqi, 2000). Heteroderidae are divided into two groups, cyst and cystoid, based on morphological alterations in their skin (Nemaplex, 2023). The cyst group are unique in that the female skin changes, transforming into a hardened cyst that protects her eggs. Unlike many plant parasites, there may be little to no aboveground evidence of an infestation with H. glycines (Constantino, 2021). They are incredibly damaging to the agricultural economy and impossible to eradicate once established. With proper management, it is possible to minimize H. glycines populations and maximize yield.
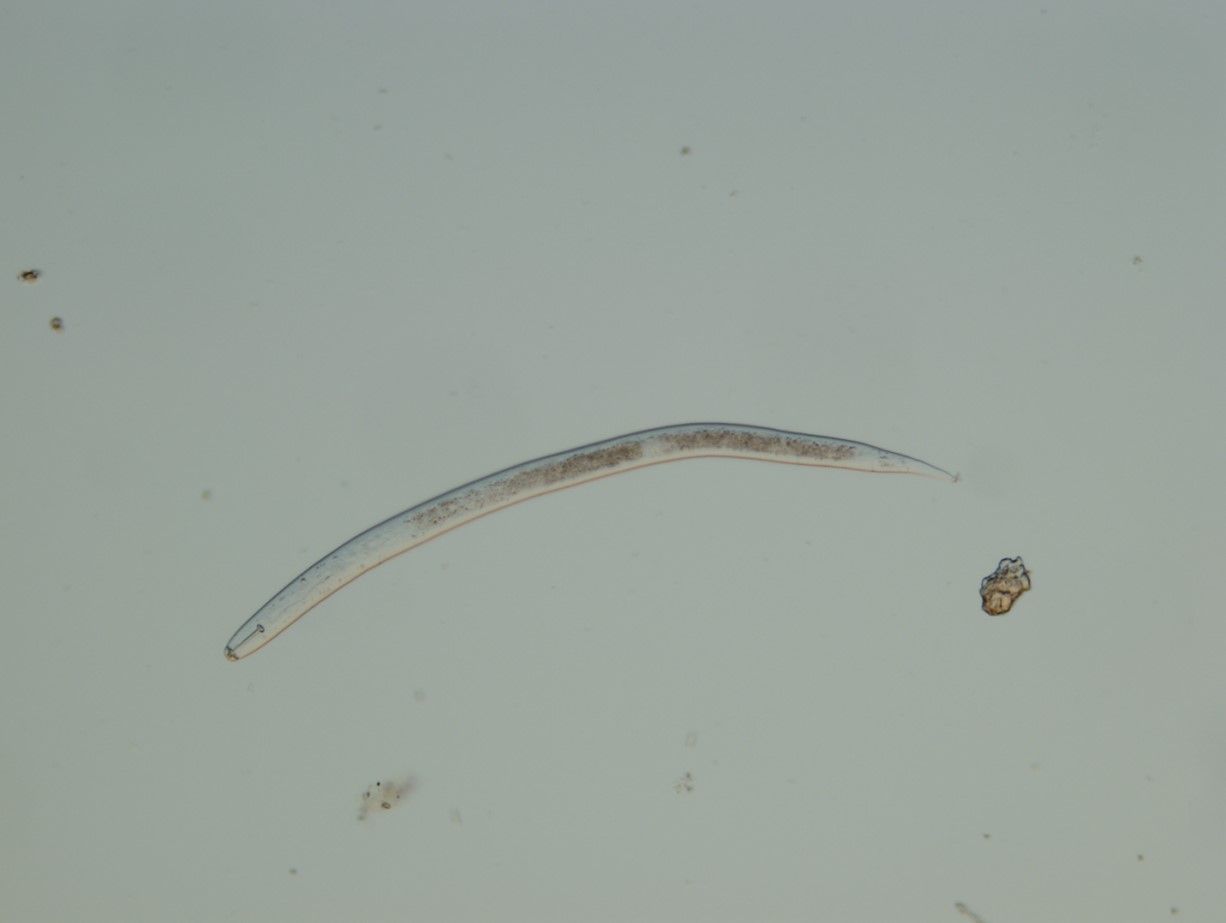
Credit: Zane Grabau, UF/IFAS
Distribution
Heterodera glycines is largely found in places where soybeans are grown commercially. Ichinohe first reported Heterodera glycines in Japan in 1915 (Riggs, 1977), though some sources reference material from 1952. It may have been previously identified and grouped as H. schachtii, which was reported in the 1880s (Ferris, 2023). As soybeans first originated in China, it is assumed that H. glycines originated there as well (Yu, 2011).
After escaping mainland China, soybean cyst nematode spread rapidly from Asia to other parts of the world through trade, wind, and animal migration. Currently, this pest is found in several countries throughout Africa, Asia, North and South America (Yu, 2011; Mesa-Valle, 2020). It also has a limited distribution in Europe, being found in 3 soybean fields in Italy in 2000 (Manachini, 2000) and 1 field each in 2018 and 2020 (Perin et al., 2021). In the United States, it is concentrated in the Midwest, Mid-South, and Southeast. H. glycines was first recognized in North Carolina in 1954 (Schmitt, 1991). Since then, it has spread to more than twenty-five states, including Florida (Figure 2).
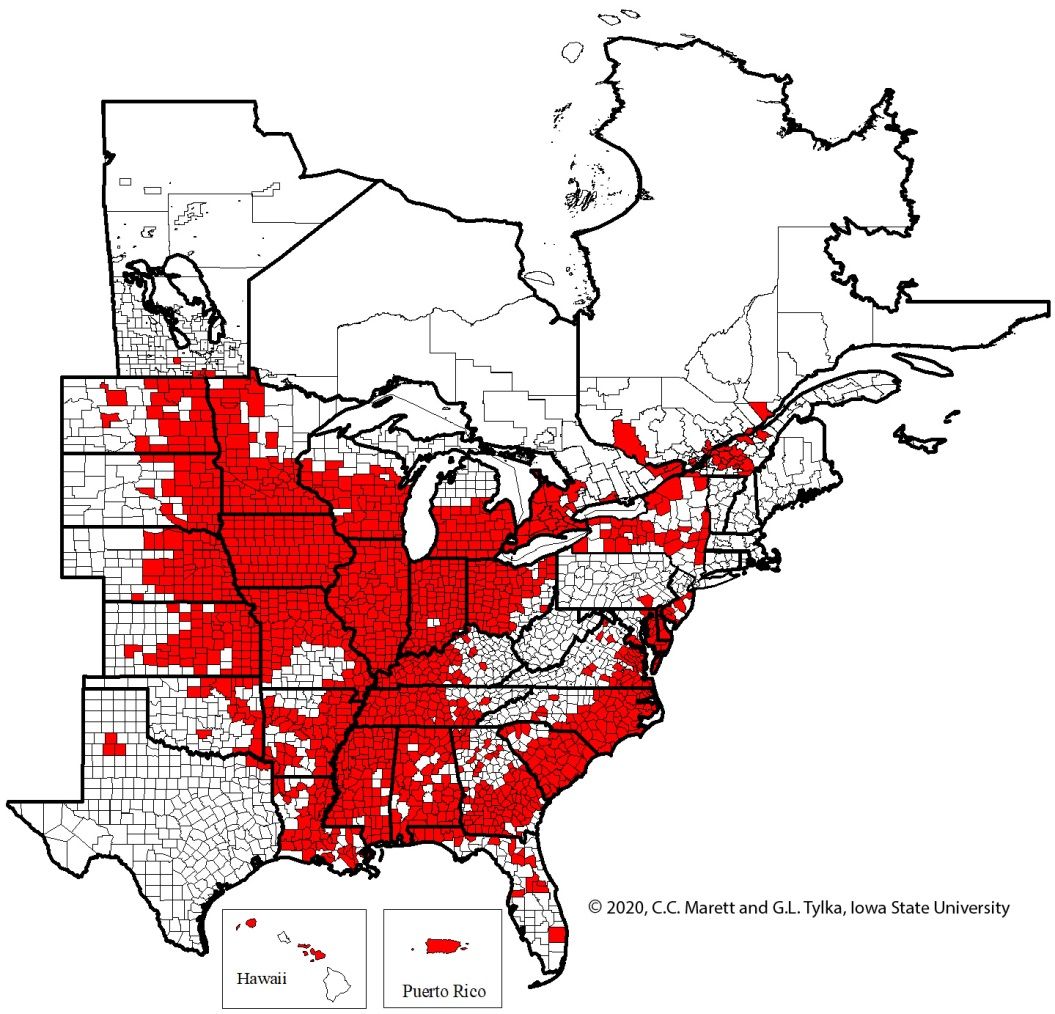
Credit: G.L. Tylka and C.C. Marett, Iowa State University. Used with permission.
Description
Heterodera glycines is a nematode that differs in morphological appearance among growth stages. They appear similar to other species in the Heterodera schachtii group. The body length of H. glycines 2nd-stage juveniles is 375–540 m with a stylet of 22.0–26.0 m. The tail measures between 40.0–61.0 m and the hyaline tail part is 21.0–33.0 m (EPPO, 2018). Stylet knobs are concave anteriorly (Figure 3). Second-stage juveniles have a vermiform shape with a sharp taper at the tail and a slight taper in the head region. The head appears offset with 3–4 annules (Figure 4). The lateral field continues to have four incisures and the tail rounded.
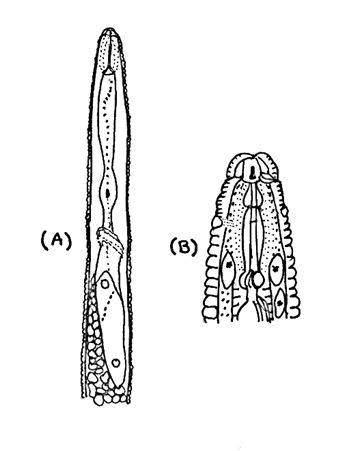
Credit: Holly Andres, University of Florida
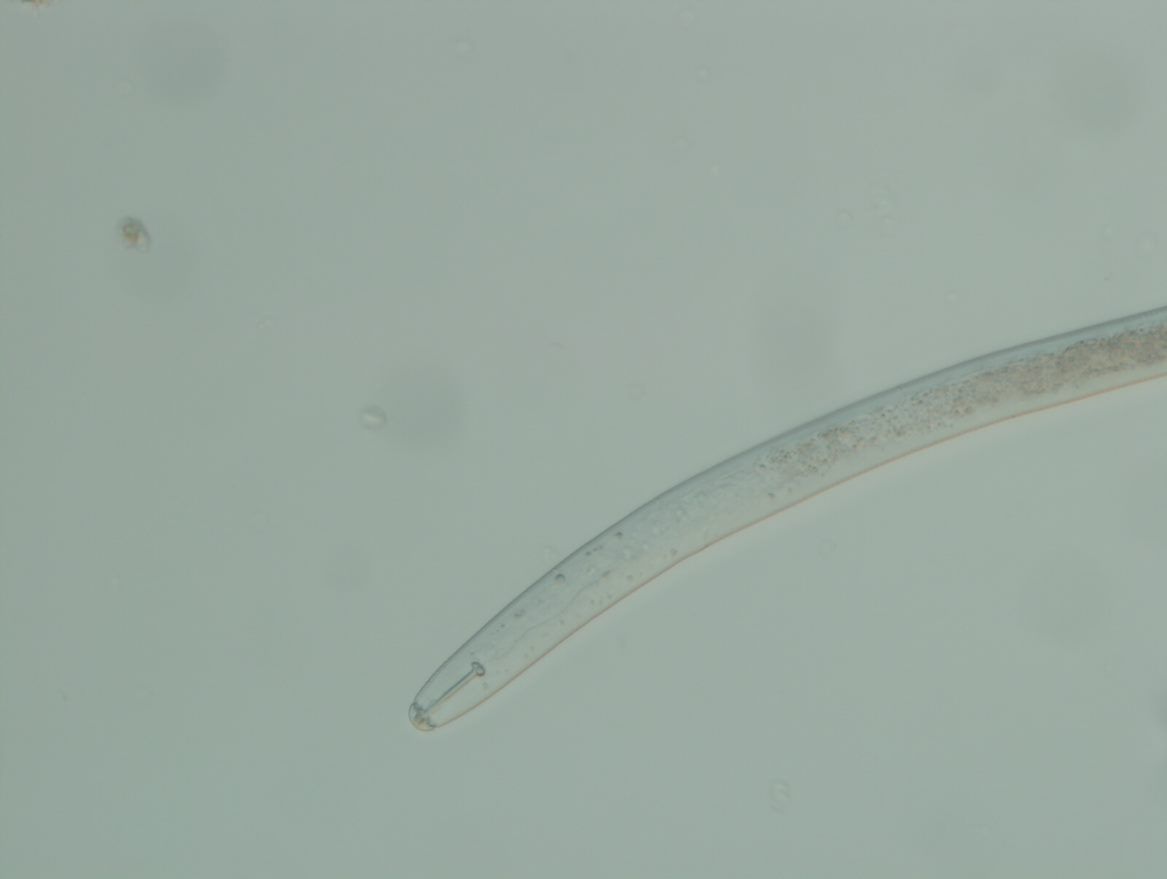
Credit: Zane Grabau, UF/IFAS
Males have a rounded tail and regular, annulated cuticle. The head has 4–5 annules and four incisures in the lateral field. Their stylet is stout with lateral knobs protruding anteriorly. The gubernaculum is present. Spicules are well formed and ventrally curved.

Credit: Zane Grabau, UF/IFAS
Females have a lemon shape and a neck that projects (Figure 5). They are white to light yellow and turn more yellow as they mature. The cysts are also shaped like a lemon with a projecting cone and neck. They are approximately 340–920 m long and 320–610 m wide. Females have reticulate ridges along their bodies with no annulation or lateral incisures. There is a prominent sub-crystalline layer. The head skeleton is hexaradiate. Females contain an egg sac that resembles a gel-like grid which holds 200–500 eggs. Their stylet is narrower with knobs that project at an angle, posteriorly. Young, sedentary females have coiled ovaries that are paired, opening posteriorly through the vulva. The vulva is enclosed by the semifenestrae, which has thin walls and a crescent shape. Bullae are distributed below the well-developed underbridge and are elongated and pronounced. In young cysts, the vulval region remains intact. When the female matures, the body wall changes from yellow to a brown cyst that encases the eggs after death. As the cyst ages, the wall becomes thin and divides, exposing an open fenestra divided by a vulval bridge (Figure 6). These are called ambifenestrate (EPPO, 2018).
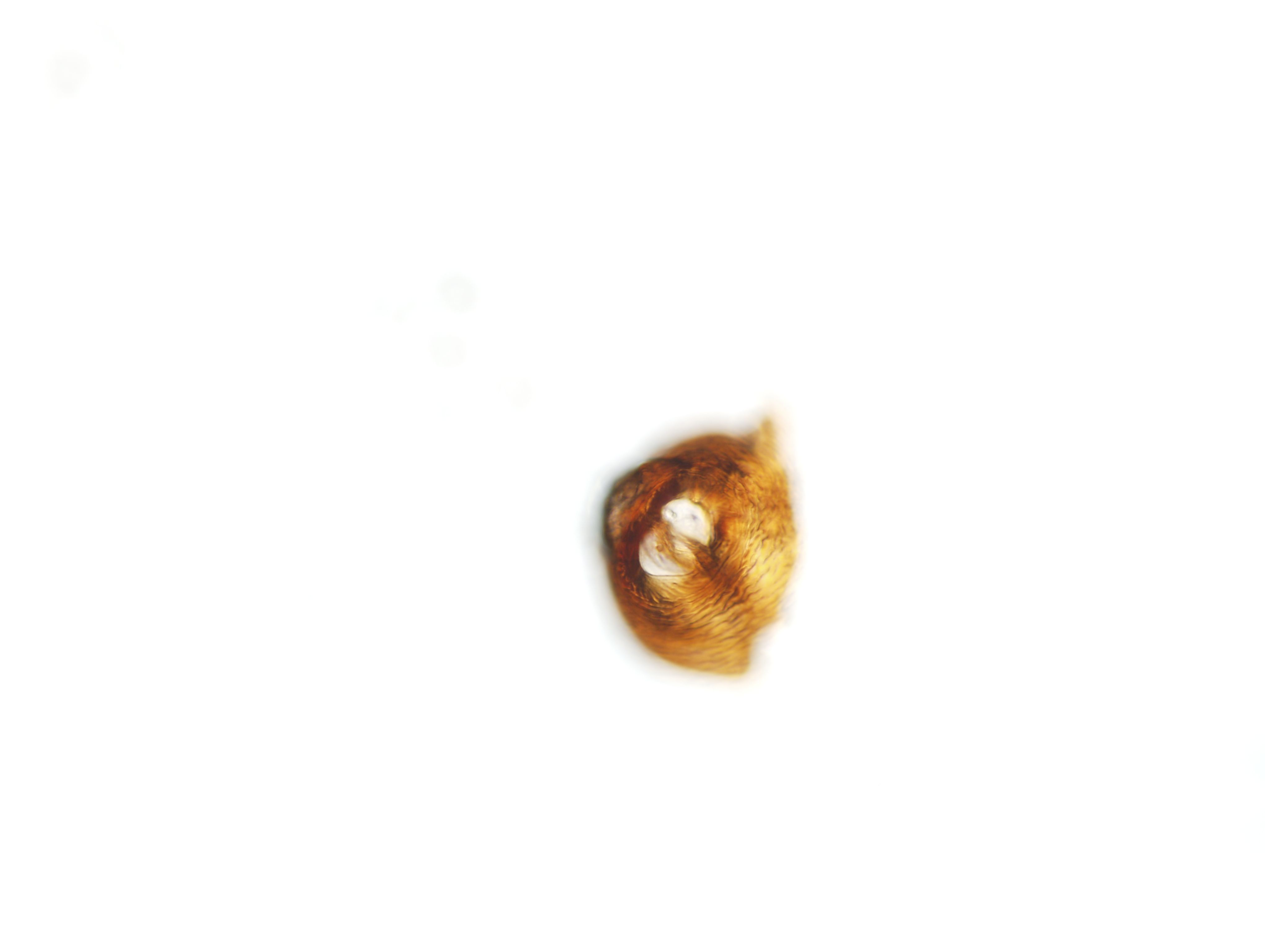
Credit: Zane Grabau, UF/IFAS
Life Cycle and Biology
The life cycle of H. glycines lasts between 3–4 weeks, should conditions be favorable (Davis and Tylka, 2021). In H. glycines, the cyst serves as a survival or overwintering structure that contains eggs. They undergo J1 molt to J2 inside the egg (Figure 7). At this point, they hatch from the egg using both a stylet and protein enzymes that help dissolve the egg wall (Gardner, 2017).
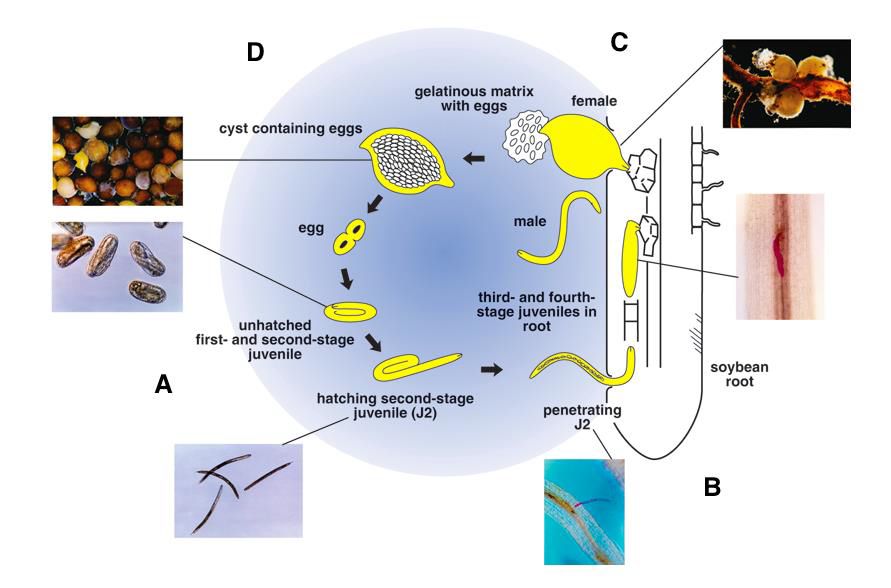
Credit: Dirk Charlson, Iowa State University. Used with permission.
Hatching is a response to chemical signaling from host plants (Giesler and Wilson, 2011). Once hatched, soybean cyst nematodes use their stylet to pierce a host plant’s root and move intra- and intercellularly toward the vasculature. Inside the vasculature, the parasite excretes effector proteins which cause a syncytium to form (Figure 8) so that the plant will nourish the parasite continuously as it loses its motility (Koenning, 2004; Gheysen and Mitchum, 2011). Inside of the syncytium, H. glycines undergoes three more molts at which point male and female adults will emerge.

Credit: Adapted from Burton Y. Endo. National Agricultural Library, Agricultural Research Service, U.S. Department of Agriculture.
SCN reproduce sexually (Koenning, 2004). Males take on a vermiform body type and exit the root to find a female for mating at which point it completes its life cycle. The female are pyriform and remain stationary. After sexual differentiation, the female begins to swell with eggs until her posterior bursts from the root and can be seen on the exterior of the host root (Figure 7) (Chowdhury et. al., 2022). Eventually the female dies; her body darkens to a brown color and hardens, encasing the eggs inside. At this point, the cysts fall off into the soil and eggs inside the cyst remain dormant until environmental cues begin the cycle again (i.e., temperature, seasonality, food resources, host plant chemical signaling) (Davis and Tylka, 2021).
Symptoms and Diagnosis
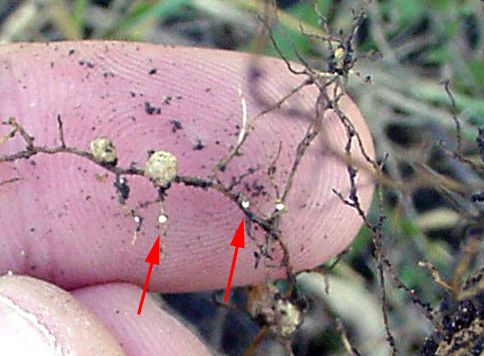
It can be difficult to diagnose an infestation since aboveground symptoms mimic many other plant ailments. Plants may not exhibit symptoms if the infection rate is low. When H. glycines have high populations within agricultural systems, plants could have chlorosis or stunted growth (Figure 9) (Musil, 2016). Often, there are no above ground symptoms and the only indication of infestation is decreased yield. Yield losses of more than 30% are possible with no visible symptoms present (Allen et. al., 2017). Belowground, lemon-shaped female cysts may be visible on roots as signs of infection and are much smaller than nitrogen-fixing soybean nodules (Figure 10). These cysts can be visible with the naked eye or more readily with a hand lens, so can be used as a preliminary diagnostic tool.

Credit: Senyu Chen, University of Minnesota. Used with permission.
Credit: Greg Tylka, Iowa State University. Used with permission.
It is important to watch fields closely to detect H. glycines infestation early. Aside from monitoring yield, assessing H. glycines in soil or root samples is necessary. The presence of H. glycines can be confirmed by extracting and quantifying H. glycines juveniles from soil using routine extraction for a variety of nematodes, such as that performed in the UF/IFAS Nematode Assay Lab. As described above, checking for cysts on gently washed plant roots (Chen et. al., 2021) is another way to confirm the presence of H. glycines. However, these methods do not always provide an accurate measurement of the severity of H. glycines infestation. In regions where H. glycines is common, quantifying cysts or eggs released from cysts from a soil sample is the recommended method for H. glycines quantification. The HG type test is also an important H. glycines diagnostic tool (Niblack et al., 2002). This test quantifies the reproduction of H. glycines from a given field on various soybean lines that serve as the sources for H. glycines resistance in commercial soybean cultivars (Niblack et al., 2002). This is an important tool for selecting a resistant soybean cultivar to grow in a given field as explained further under "Host Resistance" in the “Management” section. Professional diagnostic clinics in areas where H. glycines is common routinely provide services for HG type testing and quantification of cysts or eggs from soil.
Hosts
The primary host is soybean. Anywhere in the United States soybeans are grown, H. glycines is often present. However, H. glycines attacks several members of crops, primarily from the Fabaceae family, both ornamental and edible species, although plants from a number of other families are also hosts (EPPO, 2008; Rocha et. al., 2021). Several broadleaf species such as field pennycress, common mallow, Canada thistle, common cocklebur have been reported as potential hosts in the Midwest (Johnson et. al., 2008; Venkatesh et. al., 2000; Creech et. al., 2007; Poromarto et. al., 2015). Table 1 highlights some soybean cyst nematode hosts, roughly in order of prevalence.
Table 1. Examples of Heterodera glycines specific hosts in crop and weed species.
For crop managers it is important to know which weeds are hosts to H. glycines. Practices such as cover cropping or intercropping could potentially exacerbate the problem if a host of H. glycines is selected. Particularly in warmer regions, if winter annual weeds (hosting H. glycines) are left in crop fields, they could also increase H. glycines populations into the spring when crops are sown. This would perpetuate H. glycines life cycle through resource amplification.
Economic Importance
H. glycines is the most devastating parasite to soybean producers. It is estimated that total losses from H. glycines between 1996 and 2016 were $95.48 billion dollars (Bandara et. al., 2020). As of 2020, economic losses from H. glycines are approximately $1.5 billion each year (The SCN Coalition, 2021). H. glycines causes double the losses of all other individual diseases, comparatively on soybean crops in the United States (Allen et. al., 2017). When cultivars that are susceptible to H. glycines are selected, crop yield can decrease 60% (Hershman, 2015). In the United States, there are more than 70 million acres planted with soybean (USDA-NASS, 2023). The US is the foremost producer of soybeans globally. Soybeans comprise 90% of the US oilseed production sector (USDA-ERS, 2022). Losses in yield can be devastating to both profits and global supply chain demand.
Management
There are several categories of management practices currently utilized among growers and agricultural managers, although not each technique will be applicable in every situation. Agricultural managers may choose the best practices tailored for their needs. Some of these management practices can include: Prevention, Sanitation, Crop Rotation, Host Resistance, Nematicides, Biocontrols, and Cultural Practices.
Prevention: H. glycines persists in the soil indefinitely once it becomes established. Therefore, prevention is the best method of management. Soybean cyst nematode quarantine restricts movement of the pest from infected sites, which has been effective in certain states such as California (CDFA, 2021).
Sanitation: Closely related to prevention, sanitation practices are used to limit the movement of H. glycines since redistribution is thought to rely largely on human intervention. For example, tillage practices relocate cysts as they move soil within a field (Mangel, 2023). Soil carried on equipment can also transport H. glycines among fields, so cleaning equipment between fields can limit spread from mechanization (Ferris, 2023).
Crop Rotation: Once established in a field, current management practices involve managing this parasite to minimal numbers for maximum yield. Crop rotation with nonhost crops is part of an integrated pest management strategy to accomplish this. Since SCN is an obligate parasite, crop rotation with nonhost varieties prohibits it from reaching maturity and producing progeny (Davis and Tylka, 2021). Some nonhost crops include alfalfa, barley, canola, clover, corn, cotton, forage grasses, oats, rye, sorghum, tobacco, and wheat (Wrather and Mitchum, 2010).
Host Resistance: Another useful practice is planting resistant varieties. A single soybean line, PI 88788, has long been the predominant source of resistance in commercial soybean cultivars (Tylka, 2022). A few other sources of resistance, namely Peking, are also available in some soybean cultivars (Tylka, 2022). Because of the reliance on PI 88788-derived resistance, H. glycines populations have shifted increasingly such that populations from many fields can readily reproduce on PI 88788 cultivars (Tylka, 2022). In those fields, cultivars with PI 88788 resistance will have reduced efficacy. For this reason, rotating sources of resistance is important to delay population shifts in fields where this has not occurred. Furthermore, conducting HG type testing, as described above, is important for selecting a resistant cultivar that will be effective against the H. glycines population in a given field, particularly in areas with a history of this nematode in soybean production.
Nematicides: Some growers prefer to use nematicides to control H. glycines. Many nematicides provide temporary control; however, some are less effective due to the rapid growth cycle of H. glycines. In addition, nematicides cannot control H. glycines through the entire growing season, and infestation populations may be higher at the end of the season even with treatment (Tylka, 1994). Further information on management tips for H. glycines can be found in the UF/IFAS EDIS on nematode management in Florida soybean (Grabau, 2023).
Biocontrol: An alternative to chemical usage is biological control. Certain bacteria, fungi, and predatory nematodes can destroy H. glycines. It is important to note that only a few species are available for commercial applications, although there is continued research and progress in this field.
Cultural Practices: Controlling annual, winter weeds that host H. glycines reduces population density and subsequent pest pressure on profitable crops (Niblack and Tylka, 2008). Lastly, ensuring that plants have optimal nutrients and water will help to maintain yield and prevent susceptibility from stress. When plants are healthy and thriving, they are better able to resist infection and maintain yield.
References
Allen TW, Bradley CA, Sisson AJ, Byamukama E, Chilvers MI, Coker CM, Collins AA, Damicone JP, Dorrance AE, Dufault NS, Esker PD, Faske TR, Giesler LJ, Grybauskas AP, Hershman DE, Hollier CA, Isakeit T, Jardine DJ, Kelly HM, Kemerait RC, Kleczewski NM, Koenning SR, Jurle JE, Malvick DK, Markell SG, Mehl HL, Mueller DS, Mueller JD, Mulrroney RP, Nelson BD, Newman MA, Osborne L, Overstreet C, Padgett GB, Phipps PM, Price PP, Sikora EJ, Smith DL, Spurlock TN, Tande CA, Tenuta AU, Wise KA, Wrather JA 2017. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Progress 18(1): 19-27. https://doi.org/10.1094/PHP-RS-16-0066
Bandara AY, Weerasooriya DK, Bradley CA, Allen T, Esker PD. 2020. Dissecting the economic impact of soybean diseases in the United States over two decades. PLOS ONE 15(4): e0231141. https://doi.org/10.1371/journal.pone.0231141
California Department of Food & Agriculture (CDFA). 2021. California pest rating proposal for Heterodera glycines Ichinohe, 1952. CDFA Pest Rating Proposals and Final Ratings. (revised 22 July 2021). https://blogs.cdfa.ca.gov/Section3162/wp-content/uploads/2021/06/Heterodera_glycines_ADA_PRP-1.pdf
Chen S, Kurle J, Malvick D, Potter B, Orf J. 2021. Soybean cyst nematode management guide. University of Minnesota Extension. https://extension.umn.edu/soybean-pest-management/soybean-cyst-nematode-management-guide#management-goals-1497860
Chowdhury IA, Yan G, Halvorson J, Thapa A, Halvorson M, Markell S. 2022. Soybean cyst nematode. PP1732. North Dakota State University Extension. https://www.ndsu.edu/agriculture/extension/publications/soybean-cyst-nematode
Constantino N, Oh Y, Sennik E, Andersen B, Warden M, Oralkan Ö, Dean RA. 2021. Soybean cyst nematodes influence aboveground plant volatile signals prior to symptom development. Frontiers in Plant Science 12:749014. https://doi.org/10.3389/fpls.2021.749014
Creech JE, Johnson WG, Faghihi J, Ferris VR, Westphal A. 2005. First report of soybean cyst nematode reproduction on purple deadnettle under field conditions. Crop Management 4: 1-2. https://doi.org/10.1094/CM-2005-0715-01-BR
Creech JE, Webb JS, Young BG, Bond JP, Harrison SK, Ferris VR, Faghihi J, Westphal A, Johnson WG. 2007. Development of soybean cyst nematode on henbit (Lamium amplexicaule) and purple deadnettle (Lamium purpureum). Weed Technology 21: 1064–1070. https://doi.org/10.1614/WT-07-079.1
Davis, EL, Tylka, GL. 2021. Soybean cyst nematode disease. The Plant Health Instructor. https://doi.org/10.1094/PHI-I-2000-0725-02
Donald P, Hayes R, Walker E. 2007. Potential for soybean cyst nematode reproduction on winter weeds and cover crops in Tennessee. Plant Health Progress 8:1-6.
European and Mediterranean Plant Protection Organization (EPPO). 2018. PM 7/89 (2) Heterodera glycines. EPPO Bulletin 48: 64–77. https://doi.org/10.1111/epp.12453
European and Mediterranean Plant Protection Organization (EPPO). 2008. Heterodera glycines. EPPO Bulletin 38(3): 379-389. https://doi.org/10.1111/j.1365-2338.2008.01249.x
Ferris H. 2023. Heterodera glycines. Nemaplex website, UC Davis. (revised 16 October 2023). http://nemaplex.ucdavis.edu/Taxadata/G060s4.aspx
Gardner MN. 2017. Genetic and molecular analysis of soybean cyst nematode virulence. Dissertation. University of Missouri.
Gheysen G, Mitchum MG. 2011. How nematodes manipulate plant development pathways for infection. Current Opinion in Plant Biology 14(4): 415-421. https://doi.org/10.1016/j.pbi.2011.03.012
Giesler LJ, Wilson, JA. 2011. Soybean cyst nematode: Identification and management. G1383. University of Nebraska- Lincoln Extension. https://extensionpublications.unl.edu/assets/html/g1383/build/g1383.htm
Goodey J B, Franklin MT, Hooper DJ. 1965. T. Goodey's: The Nematode Parasites of Plants Catalogued Under Their Hosts. Third Edition. Commonwealth Agricultural Bureaux, Farnham Royal, Bucks, England.
Grabau ZJ. 2023. Management of plant parasitic nematodes in soybean production. ENY-003. EDIS 2023. https://edis.ifas.ufl.edu/publication/NG018
Hershman DE. 2015. 2015 Soybean cyst nematode (SCN) management recommendations for Kentucky. PPFS-AG-S-24. Plant Pathology Fact Sheet. University of Kentucky. https://plantpathology.ca.uky.edu/files/ppfs-ag-s-24.pdf
Hunt D. 2008. Heterodera glycines (soybean cyst nematode). CABI Compendium. https://doi.org/10.1079/cabicompendium.27027
Johnson WG, Earl Creech J, Mock, VA 2008. Role of winter annual weeds as alternative hosts for soybean cyst nematode. Crop Management 7: 1–9. https://doi.org/10.1094/CM-2008-0701-01-RV
Koenning SR. 2004. Population biology. In: Schmitt, D. P., Riggs, R. D., & Wrather, J. A. (Eds.) Biology and Management of Soybean Cyst Nematode. Schmitt & Associates of Marceline, Marceline, Mo.
Manachini, B. 2000. First report of Heterodera glycines Ichinohe on soybean in Italy. Bolletino di Zoologia Agraria e di Bachicoltura, Serie II 32(3): 261-267.
Mangel D. 2023. Soybean cyst nematode. CROPWATCH. University of Nebraska-Lincoln Institute of Agriculture and Natural Resources. https://cropwatch.unl.edu/plantdisease/soybean/soybean-cyst-nematode
Mesa-Valle CM, Garrido-Cardenas JA, Cebrian-Carmona J, Talavera M, Manzano-Agugliaro F. 2020. Global research on plant nematodes. Agronomy 10(8): 1148. https://doi.org/10.3390/agronomy10081148
Musil KM. 2016. Evaluations of biological control agents for the management of soybean cyst nematode (Heterodera glycines) in soybean (Glycine max L. Merr.). Thesis. University of Nebraska – Lincoln.
Nemaplex. 2023. Family Heteroderidae. Nemaplex website. UC Davis. (revised 14 October 2023). http://nemaplex.ucdavis.edu/Taxadata/heteidae.htm
Niblack TL, Arelli PR, Noel GR, Opperman CH, Orf JH, Schmitt DP, Shannon JG, Tylka GL. 2002. A revised classification scheme for genetically diverse populations of Heterodera glycines. Journal of Nematology 34(4): 2379-288.
Niblack TL, Tylka GL (Eds.). 2008. SCN soybean cyst nematode management guide (5th ed.). Plant Health Initiative.
Perin S, Governatori G, Braghin A. 2021. Primo ritrovamento del nematode cisticolo della soia (Heterodera glycines) in Friuli Venezia Giulia. Notiziaro ERSA 1: 28-30. http://www.ersa.fvg.it/export/sites/ersa/aziende/in-formazione/notiziario/allegati/2021/1/8_NEMATODE.pdf
Poromarto SH, Gramig GG, Nelson BD Jr., Jain S. 2015. Evaluation of weed species from the Northern Great Plains as hosts of soybean cyst nematode. Plant Health Progress 16: 23–28. https://doi.org/10.1094/PHP-RS-14-0024
Riggs RD. 1977. Worldwide distribution of soybean-cyst nematode and its economic importance. Journal of Nematology 9(1): 34-39.
Riggs RD, Hamblen M. 1962. Soybean-cyst nematode host studies in the family Leguminosae. Arkansas Agricultural Experiment Station Bulletin 50: 1-18.
Riggs RD and Hamblen M. 1966. Additional weed hosts of Heterodera glycines. Plant Disease 50: 15.
Rocha LF, Gage KL, Pimentel MF, Bond JP, Fakhoury AM. 2021. Weeds hosting the soybean cyst nematode (Heterodera glycines Ichinohe): Management implications in agroecological systems. Agronomy 11(1): 146. https://doi.org/10.3390/agronomy11010146
Schmitt DP. 1991. Management of Heterodera glycines by cropping and cultural practices. Journal of Nematology 23(3): 348–352.
The SCN Coalition. 2021. SCN keeps spreading, economic losses increasing. Blog. (released 25 January 2021). https://www.thescncoalition.com/news/2021/01/25/scn-economic-loss/
The SCN Coalition. 2023. SCN is the North America’s most damaging soybean pathogen. Let’s Talk Todes Video Library. (released 20 January 2023). https://www.thescncoalition.com/lets-talk-todes/
Siddiqi MR. 2000. Tylenchida: Parasites of Plants and Insects (2nd ed.). CABI, Wallingford, UK. https://doi.org/10.1079/9780851992020.0000
Tylka G. 1994. Soybean cyst nematode. Iowa State University Extension. https://nematode.unl.edu/scn/scnisu.htm
Tylka G. 2022. More soybean varieties with Peking SCN resistance for 2023, but more needed. Integrated Crop Management News. Iowa State University. (released 22 November 2022). https://crops.extension.iastate.edu/cropnews/2022/11/more-soybean-varieties-peking-scn-resistance-2023-more-needed
https://doi.org/10.37578/QACM6312
USDA-NASS. 2023. Crop production: 2022 Summary (January 2023). USDA-NASS, Washington DC. https://downloads.usda.library.cornell.edu/usda-esmis/files/k3569432s/9306v916d/wm119139b/cropan23.pdf
USDA-ERS. 2022. Oil crops sector at a glance. USDA-ERS website. (updated 23 October 2023). https://www.ers.usda.gov/topics/crops/soybeans-and-oil-crops/oil-crops-sector-at-a-glance/
Venkatesh R, Harrison SK, Riedel RM. 2000. Weed hosts of soybean cyst nematode (Heterodera glycines) in Ohio. Weed Technology 14: 156–160. https://doi.org/10.1614/0890-037X(2000)014[0156:WHOSCN]2.0.CO;2
Wrather A, Mitchum M. 2010. Soybean cyst nematode: Diagnosis and management. Publication No. G4450. University of Missouri Extension. https://extension.missouri.edu/publications/g4450
Yu Q. 2011. Soybean cyst nematode (Heterodera glycines Ichinohe). In: Soybean Physiology and Biochemistry. El-Shemy H. (Ed.). IntechOpen, London, UK. https://doi.org/ 10.5772/17649
Zhou Y, Wang Y, Zhu X, Liu R, Xiang P, Chen J, Liu X, Duan Y, Chen L. 2017. Management of the soybean cyst nematode Heterodera glycines with combinations of different rhizobacterial strains on soybean. PloS one, 12(8): e0182654. https://doi.org/10.1371/journal.pone.0182654