Introduction
The striped sod webworm, Fissicrambus mutabilis (Clemens), from the adult changeable grass-veneer, is a thatch-inhabiting larval pest that feeds on the aboveground stems and leaves of turfgrasses and other plants within the Gramineae (Hodgson and Roe, 2007; Vittum, 2020). This species can be found throughout eastern North America and is one of the most abundant members of the crambid moths (Vittum, 2020). Although outbreaks are rare, if large populations are uncontrolled or a pest complex is formed, this species can significantly reduce turfgrass aesthetics and cause economic damage to wheat and corn crops (Ainslie, 1923; Matheny and Heinrichs, 1975; Tofangsazi et al. 2014). Due to its abundance and behavior, it is important to monitor the species to prevent aesthetic or economic thresholds from being reached.

Credit: Keshava S R Mysore, iNaturalist, licensed under CC BY-NC-SA 4.0
Distribution
Fissicrambus mutabilis is a temperate species that is native to eastern North America. It occurs from Quebec to the north, Florida to the south, and Utah to the west, but is most abundant between Illinois, Tennessee, and Pennsylvania (Figure 2) (Fernald, 1896; Ainslie, 1923; Milne and Milne, 1980; Hodgson and Roe, 2007; Austin, 2010; Tofangsazi et al. 2014; Vittum, 2020).
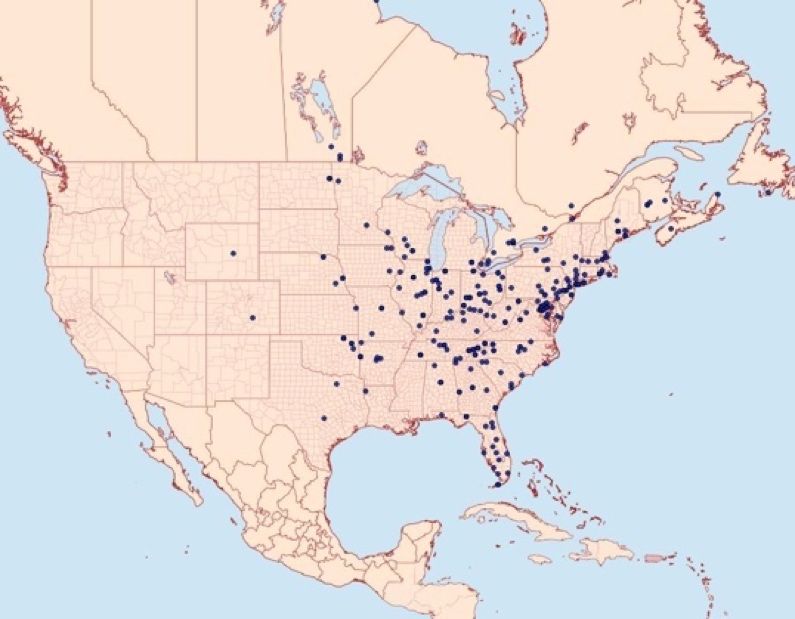
Credit: Moth Photographers Group, Mississippi State University. Used with permission.
Description
Adults
Fissicrambus mutabilis adults are part of microlepidoptera with an average wingspan of 18–24 mm (Milne and Milne, 1980; Vittum, 2020). Females are larger than males (Ainslie, 1923). Wing features seen in Figure 3 show the species’ forewings to be a pale beige-gray color with dusky spots near the center, a brown streak along the anterior proximal half, and fringes along the wing margin (Fernald, 1896; Ainslie, 1923; Milne and Milne, 1980). The hind wings are pale gray, also with fringed margins (Fernald, 1896; Ainslie, 1923). At rest, the wings curl and fold around the body giving a cylindrical appearance (Ainslie, 1923; Milne and Milne, 1980). The antennae are setaceous (taper from a thick base to a slender tip) in females and unipectinate (comb-like) in males (Ainslie, 1923). The labial palpi form a snout-like projection characteristic of crambid moths (Ainslie, 1923; McCarty, 2018; Vittum, 2020).

Credit: James F. Steffen, BugGuide. Used with permission.
Eggs
Fissicrambus mutabilis eggs are small (approximately 0.5 mm in length and 0.3 mm in width), oval-shaped, and do not stick to a substrate (Fernald, 1896; Ainslie, 1923; Vittum, 2020). They are a pale cream color when laid and deepen to a pale yellow-orange color over three days until hatching (Fernald, 1896; Ainslie, 1923).
Larvae
Fissicrambus mutabilis larvae have chewing mouthparts and undergo six to eight instars (Ainslie, 1923; Vittum, 2020). The larvae can grow up to 20 mm in length with first instars averaging 1.8 mm in length and seventh instars averaging 18 mm in length (Ainslie, 1923). The first three instars are characterized by a shining black head capsule, a pale yellow or transparent body, and dusky pinacula (small, chitinized plates) lining the dorsal and lateral cuticle (Ainslie, 1923). Later instars feature a dark yellow head capsule, a body color shifting to brown, and increasingly conspicuous longitudinal rows and pinacula also on the dorsal and lateral cuticle (Fernald, 1896; Ainslie, 1923). Fissicrambus mutabilis larvae strongly resemble the larvae of other temperate sod webworm species (Figure 4), so adults are typically used for species identification (McCarty, 2018).

Credit: Lyle Buss, UF/IFAS
Pupae
Fissicrambus mutabilis pupae are golden yellow in color and encased by cocoons located close to the ground surface (Ainslie, 1923). Cocoons are made from silk and earth particles and are approximately 15 mm in length and 7 mm in width (Ainslie, 1923).
Biology and Behavior
Fissicrambus mutabilis is a multivoltine species active between the early spring and late summer–early fall in locations north of Florida (Ainslie, 1923; Matheny and Heinrichs, 1975; Milne and Milne, 1980; Niemczyk et al. 2000). In Florida, the species is active year-round (Figure 5). The greatest flight activity occurs during the summer with peak flight periods in June and August (Matheny and Heinrichs, 1975; Niemczyk et al. 2000). Adults and larvae are active at night and can be found proximal to each other. The adults fly low to the ground within turfgrass, meadow, or crop foliage while the larvae are found in silken burrows around the stems of host plants where they hide during the day and feed at night (Ainslie, 1923; Milne and Milne, 1980; Vittum, 2020).
Table 1. Monthly observations of Fissicrambus mutabilis (Clemens) by state, 2013.
Life Cycle
Fissicrambus mutabilis produces two to three active generations per year depending on climatic conditions (Ainslie, 1923; Milne and Milne, 1980). Each female can lay up to 500 eggs which are dropped either mid-flight or at rest onto turfgrass (Ainslie, 1923; Hodgson and Roe, 2007). Eggs hatch into larvae after an average of seven days (Ainslie, 1923). The larvae develop through six to eight instars within about 36 days and pupate (Ainslie, 1923). Pupation lasts an average of seven to ten days and adults live up to two weeks (Ainslie, 1923; Milne and Milne, 1980; Vittum, 2020).
A partial generation starts in the fall and the larvae overwinter within their burrows (McCarty, 2018). The larvae then pupate in the spring, emerge as adults, and mate. The new progeny develop over the summer and create the second generation between July and August (Ainslie, 1923). In cooler locations (Figure 5), the second generation will hatch and the larvae will overwinter until the following spring (Ainslie, 1923). In warmer locations, the second generation will emerge as adults between October and November, mate, and create the third generation which will overwinter as larvae (Ainslie, 1923).

Credit: Yann Kemper, iNaturalist, licensed CC0 1.0
Host Plants
Fissicrambus mutabilis larvae feed predominantly on cool-season grasses or plants and crops within the family Graminae (Niemczyk et al. 2000). Preferred hosts include the cool-season turfgrass species Kentucky bluegrass (Poa pratensis Linnaeus) and tall fescue (Festuca aruninacea Schreber) (Vittum, 2020). Other cool-season grass hosts include perennial ryegrass (Lolium perenne Linnaeus) and bentgrass (Agrostis canina Linnaeus) (Hodgson and Roe, 2007; Vittum, 2020). Fissicrambus mutabilis larvae also feed on crops, including corn (Zea mays Linnaeus), wheat (Triticum aestivum Linnaeus), rye (Secale cereale Linnaeus), oats (Avena sativa Linnaeus), timothy (Phleum pratense Linnaeus), and clover (Trifolium species) (Pass, 1966; Hodgson and Roe, 2007; Rogers, 2014; Vittum, 2020).
Economic Importance
The turfgrass industry is valued at 60 billion dollars in the United States and makes up a large portion of the entire green industry estimated at 160 billion dollars (Braun et al. 2023). Although the specific economic impact of Fissicrambus mutabilis has not been determined, infestations negatively affect the aesthetic and economic value of turfgrasses and important crops.
Preferential host plants such as Kentucky bluegrass (Poa pratensis Linnaeus), is the most common cool-season turfgrass planted in the United States (Vittum, 2020; Shaddox et al. 2023). Other preferential turfgrass species such as ryegrasses (Lolium species) are commonly used to overseed warm-season turfgrasses (Ricketts, 2021; Wallau et al. 2023). The year-around activity of Fissicrambus mutabilis in Florida (Figure 5) is also important as the turfgrass industry has an output revenue value at 14.3 billion dollars in the state (Khachatryan et al. 2020). The species abundance, widespread distribution, and ability to form pest complexes with other crambid or turf-infesting insects make this species economically important (Ainslie, 1923; Pass, 1966; Matheny and Heinrichs, 1975; Austin, 2010; Rogers, 2014; Tofangsazi et al., 2014; Vittum, 2020).
Damage
The first three instars of Fissicrambus mutabilis feed on the surface tissues of host plants (Ainslie, 1923; Vittum, 2020). Later instars intake much more plant material from the stems and leaves of host plants and drag them down into their burrows (Ainslie, 1923; Vittum, 2020). This is similar to other sod webworm species. Damage is most apparent in late summer, beginning with a general thinning of turfgrass and increases to irregular brown patches (Ainslie, 1923; Hodgson and Roe, 2007; Rogers, 2014, Vittum, 2020). The damage may either be due to an increase in population size or to maturing larvae as damage increases with instar stage (Ainslie, 1923; Vittum, 2020).
Damage can be exacerbated through pest complexes or drought conditions. Fissicrambus mutabilis may form pest complexes with other sod webworm species including bluegrass webworms (Parapediasia teterrellus Zincken) and larger sod webworms (Pediasia trisecta Walker) (Tofangsazi et al. 2014; Vittum, 2020). Drought-induced dormancy in host plants also increases their susceptibility to damage as Fissicrambus mutabilis larvae eat more plant material under dry conditions (Vittum, 2020).

Credit: Michigan State University. Used with permission.
Management
Monitoring
Monitoring and management of Fissicrambus mutabilis are similar to other temperate sod webworm species and should focus on the presence of larvae or specific damage. Scouting should occur between seasonal flight periods in the spring and late summer when larval populations are at their highest, and in the fall for overwintering larvae (Matheny and Heinrichs, 1975; Niemczyk et al. 2000).
To monitor, grass should be parted in several areas down to the thatch and examined for silken burrows, larvae, frass, and leaf notching. Soap flushes can also be used to bring larvae, if present, to the surface (Ainslie, 1923; Hodgson and Roe, 2007; Rogers, 2014). A solution of two tablespoons of liquid dish soap mixed with two gallons of water can be poured onto one square foot of turfgrass (Hodgson and Roe, 2007). Larvae will move to the surface rapidly and their abundance can be recorded (Hodgson and Roe, 2007). Healthy turfgrass can tolerate ten to 15 larvae per square yard with minimal increases in secondary damage from birds or other predators (Hodgson and Roe, 2007; Vittum, 2020).
Biological Control
Natural enemies are successful biological controls of Fissicrambus mutabilis. Birds and arthropod predators including ground and rove beetles drastically reduce the overwintering population (Vittum, 2020). Parasites of Fissicrambus mutabilis larvae include the tachinid fly species, Phorocera claripennis (Macquart) and Aplomya caesar (Aldrich), and braconid wasps (Apanteles species) (Ainslie, 1923; Hodgson and Roe, 2007; Fernandez-Triana et al. 2014). Entomopathogenic nematodes, including Steinernema carpocapsae (Weiser) and Heterorhabditis bacteriophora (Poinar), are also successful at regulating Fissicrambus mutabilis populations (Grewal, 2001; Hodgson and Roe, 2007). Nematodes should be applied at night or in the early morning, and irrigation should occur before and after their application (Hodgson and Roe, 2007).
Cultural Control
Proper plant selection, attention to overseeding grass species, and general maintenance are sufficient for mitigating Fissicrambus mutabilis populations. Proper irrigation to avoid dry conditions can reduce rates of feeding (Hodgson and Roe, 2007; Vittum, 2020). Fertilizing turfgrass without exceeding recommended levels allows plants to outgrow damage and is useful for quick-growing and recovering turfgrasses such as Kentucky bluegrass (Hodgson and Roe, 2007; Vittum, 2020). Endophyte-infected, or endophyte-enhanced, perennial ryegrasses and fescues have resistance to sod webworms and can be used to overseed lawns (Funk et al. 1983; Hodgson and Roe, 2007; Vittum, 2020).
Chemical Control
The use of broad-spectrum insecticides should be minimized as they can reduce natural enemy populations (Hodgson and Roe, 2007). Products should be used strictly according to their labels and after other control methods have been attempted. Applications are best when larvae are young and during evenings. Biorational products such as Bacillus thuringiensis var. kurstaki can be used in the control of Fissicrambus mutabilis populations (Hodgson and Roe, 2007).
Selected References
Ainslie GG. 1923. Striped sod webworm, Crambus mutabilis Clemens. Journal of Agricultural Research 24: 399–414.
Austin GT. 2010. Moth community from a northcentral Florida location – a taxonomic checklist. Tropical Lepidoptera Research 20: 41–44.
Braun RC, Straw CM, Soldat DJ, Bekken MAH, Patton AJ, Lonsdorf EV, Horgan BP. 2023. Strategies for reducing inputs and emissions in turfgrass systems. Crop Forage & Turfgrass Management 9: e20218. https://doi.org/10.1002/cft2.20218
Fernald CH. 1896. Crambus mutabilis, pp. 57–58. In The Crambidae of North America. University of Massachusetts Amherst campus, MA. https://doi.org/10.5962/t.173040
Fernandez-Triana J, Shaw MR, Cardinal S, Mason PG. 2014. Contributions to the study of the Holarctic fauna of Microgastrinae (Hymenoptera, Braconidae). I. Introduction and first results of transatlantic comparisons. Journal of Hymenoptera Research 37: 61–76. https://doi.org/10.3897/jhr.37.7186
Funk CR, Halisky PM, Johnson MC, Siegel MR, Stewart AV, Ahmad S, Hurley RH, Harvey IC. 1983. An endophytic fungus resistance to sod webworms: association in Lolium perenne L. Nature Biotechnology 1: 189–191. https://doi.org/10.1038/nbt0483-189
Grewal P. 2001. Using entomopathogenic nematodes for turfgrass pest management. TurfGrass Trends 10: 1–7.
Haydu JJ, Hodges AW, Hall CR. 2006. Economic impacts of the turfgrass and lawncare industry in the United States. EDIS. https://fred.ifas.ufl.edu/pdf/economic-impact-analysis/FE63200.pdf (17 November 2023). https://doi.org/10.32473/edis-fe632-2006
Hodgson EW, Roe AH. 2007. Sod webworms. Utah State University Extension.
Khachatryan H, Hodges AW, Rihn A, Wei X. 2020. Economic contributions of the turfgrass industry in Florida in 2019, project report to the Florida Turfgrass Association. https://fred.ifas.ufl.edu/pdf/economic-impact-analysis/FL-TurfgrassEconomicContributions2019FinalReport.pdf (05 December 2023).
McCarty LB. 2018. Temperate sod webworms, pp. 547–548. In Golf Turf Management, CRC Press, Boca Raton, FL. https://doi.org/10.1201/9781351057950
Matheny EL, Heinrichs EA. 1975. Seasonal abundance and geographical distribution of sod webworms (Lepidoptera: Pyralidae: Crambinae) in Tennessee. Journal of the Tennessee Academy of Science 50: 33–35.
Milne LJ, Milne M. 1980. Sod webworm moths, p. 762. In National Audubon Society Field Guide to Insects & Spiders. Alfred A. Knopf, New York, NY.
Niemczyk HD, Shetlar DJ, Power KT, Richmond DS. 2000. Seasonal occurrence of the sod webworm moths (Lepidoptera: Crambidae) of Ohio. The Great Lakes Entomologist 33:173–185. https://doi.org/10.22543/0090-0222.2021
Pass BG. 1966. Control of the sod webworms Crambus teterrellus, C. trisectus, and C. mutabilis, in Kentucky. Journal of Economic Entomology 59: 19–21. https://doi.org/10.1093/jee/59.1.19
Ricketts G. 2021. Florida turfgrass selection. EDIS. https://blogs.ifas.ufl.edu/stlucieco/2021/06/09/florida-turfgrass-selection/ (16 November 2023).
Rogers DA. 2014. Crambinae (Crambidae: Lepidoptera) of Ohio: characterization, host associations and revised species accounts. M.S. thesis, Ohio State University.
Shaddox TW, Unruh JB, Johnson ME, Brown CD, Stacey G. 2023. Turfgrass use on US golf courses. HortTechnology 33: 367–376. https://doi.org/10.21273/HORTTECH05238-23
Tofangsazi N, Cherry RH, Meagher RL, Arthurs SP. 2014. Tropical sod webworm (Lepidoptera: Crambidae): a pest of warm season turfgrasses. Journal of Integrated Pest Management 5: C1–C8. https://doi.org/10.1603/IPM14014
Vittum PJ. 2020. Lepidopteran Pests: Family Crambidae (Formerly Pyralidae), Subfamily Crambinae, pp. 107–126. In Turfgrass Insects of the United States and Canada, 3rd ed. Cornell University Press, Ithaca, NY. https://doi.org/10.7591/cornell/9781501747953.003.0008
Wallau M, Blount AR, Rios E, Vendramini MB, Dubeux JCB, Babar MA, Kenworthy KE, Quesenberry KH. 2023. 2023 cool-season forage variety recommendations for Florida. EDIS. https://edis.ifas.ufl.edu/publication/AA266 (16 November 2023). https://doi.org/10.32473/edis-aa266-2023