Introduction
Viruses can have a significant negative economic impact on the international and domestic apiary industries. Globally, more than 70 viruses have been associated with Apis mellifera, the western honey bee (hereafter honey bee). Of these viruses, only a small proportion causes noticeable adverse clinical signs (Beaurepaire et al. 2020). It is common for an individual bee to be infected by multiple viruses without obvious consequence, while an entire colony can be affected by even more viruses (Ribière et al. 2008). Often, the impact from these viruses is restricted by individual-level or colony-level defenses (Chen and Siede 2007).
Honey bees demonstrate multi-tiered immune responses, individually and colony-wide, when challenged by viruses. Individually, a bee’s physiological and cellular response limits the impact of potential pathogens (Brutscher and Flenniken 2015; Maori et al. 2009). A colony’s collective response may include brood-comb fever (Starks et al. 2000), altruistic self-removal of adult bees (Rueppell et al. 2010), culling and removing infected brood via hygienic behavior (Mondet et al. 2016).
Despite these defenses, external stressors such as elevated mite infestation levels (Traynor et al. 2020), inadequate nutrition (DeGrandi-Hoffman and Chen 2015), or human-caused disturbance of the surrounding ecology (McMahon et al. 2018) can allow viruses to cause significant damage. In fact, the significance of viruses to colony health has been demonstrated by studies showing strong correlations between viral infection and colony loss (Cox-Foster et al. 2007; Grozinger and Flenniken 2019). The purpose of this article is to provide beekeepers an overview of bee viruses commonly found in Florida, virus impact on colony health, associated clinical signs, and transmission pathways (Table 1).
Biology
Viruses are invisible to the naked eye. Under an electron microscope, viral particles are found in many shapes, including rods, spheres, and filaments. They are comprised of nucleic acids (single-stranded or double-stranded RNA or DNA) and a protein coat. Viruses are composed of compact genomes; therefore, they are entirely dependent on their host for replication. They repurpose host cellular machinery to produce their own components. Most described honey bee viruses are RNA viruses; however, a few DNA viruses have also been reported to be associated with honey bees (Amiri et al. 2021).
Clinical Signs
Viruses are often present without causing obvious distress in infected honey bees. In other cases, infections can persist subclinically as chronic disease. When clinical signs are present, they may include physical deformities, paralysis, trembling, shortened lifespans, abrupt mortality, cuticular (body) discoloration, and cognitive impairments. Each virus causes unique disease expression across the various bee life stages (brood, adult, etc.), and the prognosis of an individual bee can vary from no clinical signs to death within a few days.
Diagnosis
For honey bee viruses, detection and diagnosis are confirmed using two main techniques: enzyme-linked immunosorbent assay (ELISA) or reverse-transcriptase (quantitative) polymerase chain reaction [RT-(q)PCR]. ELISA is a protein-based serological technique that uses antibodies to detect the presence or absence of a disease target. RT-(q)PCR is a nucleic-acid-based technique. In this process, RNA is converted into single-stranded complementary DNA (cDNA), which is then used in the PCR. For conventional RT-PCR, the product is visualized on an agarose gel to determine presence or absence of the target gene. For RT-(q)PCR, cDNA amplification is visualized on a computer, and viral loads can be quantified in real time. Because these techniques require trained laboratory technicians and specific equipment, sending suspect samples to one of the honey bee diagnostic laboratories in North America is recommended. ApiaryInspectors.org has a list of apiary inspectors of America.
Epidemiology
Honey bee viruses can spread by various mechanisms, including from queen to egg, orally during trophallaxis (feeding one another), by exposure to fecal material, and by bodily contact. However, one of the most significant factors contributing to the heightened impact of viruses to apiary health is the presence of Varroa destructor, a parasitic mite that feeds and reproduces on honey bees. In addition to causing direct damage to honey bee tissue, predation by V. destructor also depresses honey bee immunity, impairs detoxification processes, and causes malnutrition as the honey bee physiology responds to constant predation (Morfin et al. 2023). These detrimental effects are aggravated by V. destructor’s capacity to vector several honey bee viruses (Damayo et al. 2023). In fact, the spread of V. destructor has caused significant increases in the number and diversity of viruses afflicting individuals and colonies (Doublet et al. 2024). The impact of each virus depends largely on whether the virus is vector transmitted.
Management
Unfortunately, no chemical applications are available for the direct treatment of viral diseases of honey bees. Instead, beekeepers are encouraged to maintain conscientious beekeeper practices to ensure that colonies are successful (Bartlett et al. 2021).
- Varroa destructor is responsible for transmitting several honey bee viruses and weakens honey bee populations through parasitism. Consequently, adept use of acaricides and integrated pest management strategies that keep populations of V. destructor low/controlled is an essential component of any disease-management program (Locke et al. 2017; Jack and Ellis 2021). See also “Varroa Management,” available at HoneyBeeHealthCoalition.org, for information related to controlling V. destructor in honey bee colonies.
- Ensure colonies have access to adequate nutrition (DeGrandi-Hoffman et al. 2010). Access to an abundance of polyfloral pollen will support colony health during viral exposures (Dolezal et al. 2019).
- When choosing queen stock, choose lineages that possess innate resistance or enhanced altruistic behaviors such as self-removal or diseased/dead brood removal, i.e., bees that express hygienic behavior (Wagoner et al. 2019).
- Manage for other pathogens and pests, including Melissococcus plutonius (causative agent of European foulbrood) and small hive beetles (Aethina tumida), to reduce colony stress, thus likely lessening the impact of viral disease.
- Provide a hygienic environment by sterilizing hive tools, replacing worn and/or degraded hive equipment, and limiting material transfer between apiaries.
- Quarantine and remove or sanitize infectious material (e.g., used frames, dirty extraction equipment, etc. [de Miranda 2012]) to interrupt viral epidemics. For example, if a particular colony develops severe disease pressure, then that colony can be removed from an apiary and the constituent hive material (i.e., frame, foundation, etc.) sanitized and stored until the following season.
- Minimize pesticide exposure, because viral lethality and honey bee susceptibility to infection have been correlated with pesticide exposure (McMenamin et al. 2016).
Common Florida Honey Bee Viruses
Knowledge and understanding of honey bee viruses in Florida are vital for the overall health of these pollinators. Within Florida, 14 viruses are routinely detected, with eight of those posing a particular threat to the industry (Table 1). Common honey bee viruses include acute bee paralysis virus (ABPV), Kashmir bee virus (KBV), Israeli acute paralysis virus (IAPV), black queen cell virus (BQCV), chronic bee paralysis virus (CBPV), deformed wing virus (DWV), Lake Sinai virus (LSV), and sacbrood virus (SBV). Detection and diagnosis allow for proper management techniques as well as stronger colonies.
Acute Bee Paralysis Virus/Kashmir Bee Virus/Israeli Acute Paralysis Virus
Closely related viruses ABPV, KBV, and IAPV share many characteristics that form the acute bee paralysis virus–Kashmir bee virus–Israeli acute paralysis virus complex in the single-stranded RNA Dicistroviridae virus family (de Miranda et al. 2010). Acute bee paralysis virus is often found in honey bee colonies without overt clinical signs and is possibly spread through trophallaxis (Yañez et al. 2020). When V. destructor populations become large in a colony, this virus can lead to high bee mortality rates (Bakonyi et al. 2002). Furthermore, ABPV is implicated in colony collapse (Šimenc et al. 2021). Clinical signs may include hair loss and cuticular darkening in adult bees (Amiri et al. 2021) (Figure 1).

Credit: UF/IFAS Honey Bee Research and Extension Lab
Kashmir bee virus can infect both brood and adult honey bees. Infected adults often quickly die after inoculation. Low levels of infection are known in the absence of V. destructor but can reach epidemic levels when mite populations are high (Chen et al. 2004). Overt signs besides death are not apparent (Riveros et al. 2018).
Similar to ABPV and KBV, IAPV can be spread through trophallaxis and V. destructor infestation (Chen et al. 2014). Clinical signs can vary from asymptomatic infection to trembling, paralysis, death (Formato et al. 2011), and behavioral changes (Geffre et al. 2020), ultimately leading to high colony losses (Maori et al. 2009).
Black Queen Cell Virus
Black queen cell virus is another member of the Dicistroviridae family of RNA viruses. As the name suggests, one of the most visible clinical signs associated with this pathogen is the darkening of dead queens in queen cells (Figure 2). The immature queens developing in these cells die from infection and turn black. Black queen cell virus is one of the most common honey bee viruses and can be found globally (Tentcheva et al. 2004). It is spread through trophallaxis, vertical transmission from queen to eggs, and in worker-produced royal jelly (Chen et al. 2006). In adult bees, this pathogen is found in co-infection with Nosema apis and Malpighamoeba mellificae (Bailey et al. 1983).

Credit: UF/IFAS Honey Bee Research and Extension Laboratory
Chronic Bee Paralysis Virus
Chronic bee paralysis virus is a global disease of increasing significance (Budge et al. 2020). Two sets of clinical signs exist, Type 1 and Type 2 (Ribière et al. 2010). Type 1 infection is categorized by flightless bees with bloated abdomens and trembling wings (Figure 3). Type 2 infection consists of darkened, shiny bees that appear greasy and hairless. Initially these bees can fly, but after a few days they lose the ability to fly. Inoculated bees survive more than 12 days (Bailey et al. 1963). This virus is spread through physical contact with infected bees and contact with contaminated feces (Amiri et al. 2014).

Credit: Professor Giles Budge, Newcastle University, used with permission
Deformed Wing Virus
Deformed wing virus is a highly common honey bee virus with four variant strains (A, B, C, and D). It is a positive, single-stranded RNA virus in the Iflaviridae family of viruses. One study demonstrated that across 32 countries, over 55% of colonies were affected by at least one strain of DWV (Martin and Brettell 2019). As the name suggests, the most obvious clinical sign of infection includes deformed wings (Figure 4). However, only about 1% of infected bees display this clinical sign (Brettell et al. 2017). Many infected bees will also present bloated abdomens, impaired cognition, erratic behavior, and shorter lifespans (Kevill et al. 2021). However, abnormalities in physiology and/or anatomy usually only occur when viral titers are extremely elevated. The virus is strongly associated with V. destructor, with regions without established mite populations having little or no DWV infections (Martin and Brettell 2019). Other forms of DWV spread include reproduction, resource sharing, and trophallaxis (Martin and Brettell 2019).

Credit: J. D. Ellis and C. M. Zettel Nalen 2022
Lake Sinai Virus
First discovered in 2008 (Runckel et al. 2011), LSV has several strains, with LSV 1 and 2 being the most common. While LSV seems to be a latent virus, producing no apparent signs of infection, there is a correlation between high viral loads and weaker colonies with low populations (Daughenbaugh et al. 2015). There is evidence that LSV is not associated with V. destructor. Its mode of infection is not clearly understood (Hou et al. 2023).
Sacbrood Virus
Sacbrood virus, an RNA virus, infects the brood of honey bees. Larvae infected with this virus die before their final molt. Fluid accumulates at the bottom of the larva as viral particles replicate in the larva and prevents it from shedding the endocuticle (Li et al. 2019; Wei et al. 2022) (Figure 5). Subsequently, this causes larval mortality, turning the larva from pale yellow to dark brown (Grabensteiner et al. 2000). The clinical signs are similar to those associated with American foulbrood (spotty brood pattern, discolored/sunken/perforated brood cell cappings). The key difference is that the dead individuals do not adhere to the cell walls. Sacbrood virus can affect adults with no obvious clinical signs of infection, but the virus can also shorten the lifespan of infected bees (Grabensteiner et al. 2000). SBV infections are spread multiple ways, including possibly through V. destructor, reproduction, and trophallaxis (Wei et al. 2022). Sacbrood is most prevalent in the spring due to changing temperatures (Li et al. 2019).
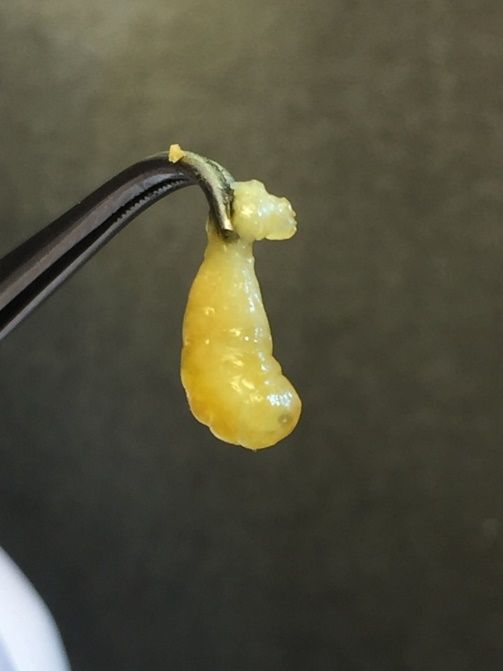
Credit: Joachim de Miranda, Swedish University of Agricultural Sciences, used with permission
Conclusion
Bee virology is a challenging field, but our knowledge of viral pathogens, including their effects on the honey bee host and ecological interactions, is constantly improving. Surveillance and monitoring are necessary components of safeguarding the industry. Florida beekeepers are encouraged to use resources available to them, including many available through their Florida Department of Agriculture and Consumer Services Division of Plant Industry. Find a local apiary inspector and/or a honey bee diagnostics laboratory, both available, along with an array of other valuable bee resources, at fdacs.gov.
References
Amiri, E., M. Meixner, R. Büchler, and P. Kryger. 2014. “Chronic Bee Paralysis Virus in Honeybee Queens: Evaluating Susceptibility and Infection Routes.” Viruses 6 (3): 1188–1201. https://doi.org/10.3390/v6031188
Amiri, E., O. Rueppell, and D. R. Tarpy. 2021. “Honey Bee Viral Diseases.” In Honey Bee Medicine for the Veterinary Practitioner, edited by T. R. Kane and C. M. Faux., pp. 253–275, John Wiley & Sons Inc. https://doi.org/10.1002/9781119583417.ch21
Bailey, L., B. V. Ball, and J. N. Perry. 1983. “Association of Viruses with Two Protozoal Pathogens of the Honey Bee.” Annals of Applied Biology 103 (1): 13–20. https://doi.org/10.1111/j.1744-7348.1983.tb02735.x
Bailey, L., A. J. Gibbs, and R. D. Woods. 1963. “Two Viruses from Adult Honey Bees (Apis mellifera Linnaeus).” Virology 21 (3): 390–395. https://doi.org/10.1016/0042-6822(63)90200-9
Bailey, L., and R. D. Woods. 1977. “Two More Small RNA Viruses from Honey Bees and Further Observations on Sacbrood and Acute Bee-Paralysis Viruses.” Journal of General Virology 37 (1): 175–182. https://doi.org/10.1099/0022-1317-37-1-175
Bakonyi, T., E. Grabensteiner, J. Kolodziejek, et al. 2002. “Phylogenetic Analysis of Acute Bee Paralysis Virus Strains.” Applied and Environmental Microbiology 68 (12): 6446–6450. https://doi.org/10.1128/AEM.68.12.6446-6450.2002
Ball, B. V., and L. Bailey. 1999. “Honey Bee Viruses.” In Encyclopedia of Virology, 2nd ed., edited by A. Granoff and R. G. Webster, pp. 743–749, Academic Press. https://doi.org/10.1006/rwvi.1999.0139
Bartlett, L. J. 2022. “Frontiers in Effective Control of Problem Parasites in Beekeeping.” International Journal for Parasitology: Parasites and Wildlife 17:263–272. https://doi.org/10.1016/j.ijppaw.2022.03.003
Bartlett, L. J., M. Boots, B. J. Brosi, et al. 2020. “Persistent Effects of Management History on Honeybee Colony Virus Abundances.” Journal of Invertebrate Pathology 179:107520. https://doi.org/10.1016/j.jip.2020.107520
Beaurepaire, A., N. Piot, V. Doublet, et al. 2020. “Diversity and Global Distribution of Viruses of the Western Honey Bee, Apis mellifera.” Insects, 11 (4): 239. https://doi.org/10.3390/insects11040239
Brettell, L. E., G. J. Mordecai, D. C. Schroeder, et al. 2017. “A Comparison of Deformed Wing Virus in Deformed and Asymptomatic Honey Bees." Insects 8 (1): 28. https://doi.org/10.3390/insects8010028
Brutscher, L. M., and M. L. Flenniken. 2015. “RNAi and Antiviral Defense in the Honey Bee.” Journal of Immunology Research 2015:941897. https://doi.org/10.1155/2015/941897
Budge, G. E., N. K. Simcock, P. J. Holder, et al. 2020. “Chronic Bee Paralysis as a Serious Emerging Threat to Honey Bees.” Nature Communications 11 (1): 2164. https://doi.org/10.1038/s41467-020-15919-0
Calderone, N. W. 2012. “Insect Pollinated Crops, Insect Pollinators and US Agriculture: Trend Analysis of Aggregate Data for the Period 1992–2009.” PLoS ONE 7 (5): e37235. https://doi.org/10.1371/journal.pone.0037235
Chen, Y. P., J. S. Pettis, A. Collins, and M. F. Feldlaufer. 2006. “Prevalence and Transmission of Honeybee Viruses.” Applied and Environmental Microbiology 72 (1): 606–611. https://doi.org/10.1128/AEM.72.1.606-611.2006
Chen, Y. P., J. S. Pettis, M. Corona, et al. 2014. “Israeli Acute Paralysis Virus: Epidemiology, Pathogenesis and Implications for Honey Bee Health.” PLoS Pathogens 10 (7): e1004261. https://doi.org/10.1371/journal.ppat.1004261
Chen, Y., J. S. Pettis, J. D. Evans, M. Kramer, and M. F. Feldlaufer. 2004. “Transmission of Kashmir Bee Virus by the Ectoparasitic Mite Varroa destructor.” Apidologie 35 (4): 441–448. https://doi.org/10.1051/apido:2004031
Chen, Y. P., and R. Siede. 2007. “Honey Bee Viruses.” Advances in Virus Research 70: 33–80. https://doi.org/10.1016/S0065-3527(07)70002-7
Cornman, R. S., D. R. Tarpy, Y. Chen, et al. 2012. “Pathogen Webs in Collapsing Honey Bee Colonies.” PLoS ONE 7 (8): e43562. https://doi.org/10.1371/journal.pone.0043562
Cox-Foster, D. L., S. Conlan, E. C. Holmes, et al. 2007. “A Metagenomic Survey of Microbes in Honey Bee Colony Collapse Disorder.” Science 318 (5848): 283–287. https://doi.org/10.1126/science.1146498
Damayo, J. E., R. C. McKee, G. Buchmann, A. M. Norton, A. Ashe, and E. J. Remnant. 2023. “Virus Replication in the Honey Bee Parasite, Varroa destructor.” Journal of Virology 97 (12): e01149–23. https://doi.org/10.1128/jvi.01149-23
Daughenbaugh, K. F., M. Martin, L. M. Brutscher, et al. 2015. “Honey Bee Infecting Lake Sinai Viruses.” Viruses 7 (6): 3285–3309. https://doi.org/10.3390/v7062772
De Miranda, J. R. 2008. “Diagnostic Techniques for Virus Detection in Honey Bees.” In Virology and the Honey Bee, edited by M. F. A. Aubert, B. V. Ball, I. Fries, R. F. A. Moritz, N. Milani, and I. Bernardinelli. pp. 121–232, European Commission.
De Miranda, J. R. 2012. “Viruses In Bees: What do they do and what can we do about it?” Bee World 89 (1): 2–5. https://doi.org/10.1080/0005772X.2012.11417446
De Miranda, J. R., G. Cordoni, and G. Budge. 2010. “The Acute Bee Paralysis Virus–Kashmir Bee Virus–Israeli Acute Paralysis Virus Complex.” Journal of Invertebrate Pathology 103: S30–S47. https://doi.org/10.1016/j.jip.2009.06.014
De Miranda, J. R., G. L. Ribière, M. Y. P. Chen. 2011. “Honey Bee Viruses and Their Effects on Bee and Colony Health.” In Honey Bee Colony Health: Challenges and Sustainable Solution, edited by D. Sammataro, and J. A. Yoder, pp. 71–102, CRC Press, Taylor & Francis Group. https://doi.org/10.1201/b11318-8
DeGrandi-Hoffman, G., and Y. Chen. 2015. “Nutrition, Immunity and Viral Infections in Honey Bees.” Current Opinion in Insect Science 10:170–176. https://doi.org/10.1016/j.cois.2015.05.007
DeGrandi-Hoffman, G., Y. Chen, E. Huang, and M. H. Huang. 2010. “The Effect of Diet on Protein Concentration, Hypopharyngeal Gland Development and Virus Load in Worker Honey Bees (Apis mellifera L.).” Journal of Insect Physiology 56 (9): 1184–1191. https://doi.org/10.1016/j.jinsphys.2010.03.017
Dolezal, A. G., J. Carrillo-Tripp, T. M. Judd, A. M. Miller, B. C. Bonning, and A. L. Toth. 2019. “Interacting Stressors Matter: Diet Quality and Virus Infection in Honeybee Health." Royal Society Open Science 6 (2): 181803. https://doi.org/10.1098/rsos.181803
Doublet, V., M. A. Y. Oddie, F. Mondet, et al. 2024. “Shift in Virus Composition in Honeybees (Apis mellifera) Following Worldwide Invasion by the Parasitic Mite and Virus Vector Varroa destructor.” Royal Society Open Science 11 (1): 231529. https://doi.org/10.1098/rsos.231529
Ellis, J. D., and C. M. Zettel Nalen. 2022. Varroa, Varroa destructor Anderson and Trueman (Arachnida: Acari: Varroidae). EENY-473. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/IN855
Formato, G., A. Giacomelli, M. Olivia, et al. 2011. “First Detection of Israeli Acute Paralysis Virus (IAPV) in Italy.” Journal of Apicultural Research 50 (2): 176–177. https://doi.org/10.3896/IBRA.1.50.2.12
Fujiyuki, T., H. Takeuchi, M. Ono, et al. 2004. “Novel Insect Picorna-Like Virus Identified in the Brains of Aggressive Worker Honeybees.” Journal of Virology 78 (3): 1093–1100. https://doi.org/10.1128/JVI.78.3.1093-1100.2004
Geffre, A. C., T. Gernat, G. P. Harwood, et al. 2020. “Honey Bee Virus Causes Context-Dependent Changes in Host Social Behavior.” Proceedings of the National Academy of Sciences 117 (19): 10406–10413. https://doi.org/10.1073/pnas.2002268117
Grabensteiner, E., W. Ritter, M. J. Carter, et al. 2001. “Sacbrood Virus of the Honeybee (Apis mellifera): Rapid Identification and Phylogenetic Analysis Using Reverse Transcription-PCR.” Clinical Diagnostic Laboratory Immunology 8 (1): 93–104. https://doi.org/10.1128/CDLI.8.1.93-104.2001
Grozinger, C. M., and M. L. Flenniken. 2019. “Bee Viruses: Ecology, Pathogenicity, and Impacts.” Annual Review of Entomology 64 (1): 205–226. https://doi.org/10.1146/annurev-ento-011118-111942
Hou, C., H. Liang, C. Chen, et al. 2023. “Lake Sinai Virus Is a Diverse, Globally Distributed but not Emerging Multi‐Strain Honeybee Virus.” Molecular Ecology 32 (14): 3859–3871. https://doi.org/10.1111/mec.16987
Hunter, W., J. Ellis, D. van Engelsdorp, et al. 2010. “Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (Apis mellifera, Hymenoptera: Apidae).” PLoS Pathogens 6 (12): e1001160. https://doi.org/10.1371/journal.ppat.1001160
Iqbal, J., and U. Mueller. 2007. “Virus Infection Causes Specific Learning Deficits in Honeybee Foragers.” Proceedings of the Royal Society B: Biological Sciences 274 (1617): 1517–1521. https://doi.org/10.1098/rspb.2007.0022
Jack, C. J., and J. D. Ellis. 2021. “Integrated Pest Management Control of Varroa destructor (Acari: Varroidae), the Most Damaging Pest of (Apis mellifera L. (Hymenoptera: Apidae)) Colonies.” Journal of Insect Science 21 (5): 6. https://doi.org/10.1093/jisesa/ieab058
Kevill, J. L., K. C. Stainton, D. C. Schroeder, and S. J. Martin. 2021. “Deformed Wing Virus Variant Shift from 2010 to 2016 in Managed and Feral UK Honey Bee Colonies.” Archives of Virology 166:2693–2702. https://doi.org/10.1007/s00705-021-05162-3
Leonard, S. P., J. E. Powell, J. Perutka, et al. 2020. “Engineered Symbionts Activate Honey Bee Immunity and Limit Pathogens.” Science 367 (6477): 573–576. https://doi.org/10.1126/science.aax9039
Li, J., T. Wang, J. D. Evans, et al. 2019. “The Phylogeny and Pathogenesis of Sacbrood Virus (SBV) Infection in European Honey Bees, Apis mellifera.” Viruses 11 (1): 61. https://doi.org/10.3390/v11010061
Locke, B., E. Semberg, E. Forsgren, and J. R. de Miranda. 2017. “Persistence of Subclinical Deformed Wing Virus Infections in Honeybees Following Varroa Mite Removal and a Bee Population Turnover.” PLoS ONE 12 (7): e0180910. https://doi.org/10.1371/journal.pone.0180910
Mallinger, R. E., J. J. Ternest, S. A. Weaver, J. Weaver, and S. Pryer. 2021. “Importance of Insect Pollinators for Florida Agriculture: A Systematic Review of the Literature.” Florida Entomology 104 (3): 222–229. https://doi.org/10.1653/024.104.0312
Maori, E., N. Paldi, S. Shafir, et al. 2009. “IAPV, a Bee‐Affecting Virus Associated with Colony Collapse Disorder Can Be Silenced by dsRNA Ingestion.” Insect Molecular Biology 18 (1): 55–60. https://doi.org/10.1111/j.1365-2583.2009.00847.x
Martin, S. J., B. V. Ball, and N. L. Carreck. 2015. “Prevalence and Persistence of Deformed Wing Virus (DWV) in Untreated or Acaricide-Treated Varroa destructor Infested Honey Bee (Apis mellifera) Colonies.” Journal of Apicultural Research 49 (1): 72–79. https://doi.org/10.3896/IBRA.1.49.1.10
Martin, S. J., L. E. Brettell. 2019. “Deformed Wing Virus in Honeybees and Other Insects.” Annual Review of Virology 6 (1): 49–69. https://doi.org/10.1146/annurev-virology-092818-015700
Mathieu, O., and J. Bender. 2004. “RNA-Directed DNA Methylation.” Journal of Cell Science 117 (21): 4881–4888. https://doi.org/10.1242/jcs.01479
McMahon, D. P., L. Wilfert, R. J. Paxton, and M. J. F. Brown. 2018. “Emerging Viruses in Bees: From Molecules to Ecology.” In Advances in Virus Research , edited by C. M. Malmstrom, Vol. 101, pp. 251–291, Academic Press, Inc. https://doi.org/10.1016/bs.aivir.2018.02.008
McMenamin, A. J., L. M. Brutscher, W. Glenny, and M. L. Flenniken. “Abiotic and Biotic Factors Affecting the Replication and Pathogenicity of Bee Viruses.” Current Opinion in Insect Science 16:14–21. https://doi.org/10.1016/j.cois.2016.04.009
Mondet, F., S. H. Kim, J. R. de Miranda, D. Beslay, Y. L. Conte, and A. R. Mercer. 2016. “Specific Cues Associated with Honey Bee Social Defence against Varroa destructor Infested Brood.” Scientific Reports 6 (1): 25444. https://doi.org/10.1038/srep25444
Morfin, N., P. H. Goodwin, E. Guzman-Novoa. 2023. “Varroa destructor and Its Impacts on Honey Bee Biology.” Frontiers in Bee Science 1:1272937. https://doi.org/10.3389/frbee.2023.1272937
Nicola Gallai, N., J.-M. Salles, J. Settele, and B. E. Vaissière. 2009. “Economic Valuation of the Vulnerability of World Agriculture Confronted with Pollinator Decline.” Ecological Economics 68 (3): 810–821. https://doi.org/10.1016/j.ecolecon.2008.06.014
Palmer-Young, E. C., C. Ö. Tozkar, R. S. Schwarz, et al. 2017. “Nectar and Pollen Phytochemicals Stimulate Honey Bee (Hymenoptera: Apidae) Immunity to Viral Infection.” Journal of Economic Entomology 110 (5): 1959–1972. https://doi.org/10.1093/jee/tox193
Pasho, D. J. M., J. R. Applegate, and D. I. Hopkins. 2021. “Diseases and Pests of Honey Bees (Apis mellifera).” Veterinary Clinics: Food Animal Practice 37 (3): 401–412. https://doi.org/10.1016/j.cvfa.2021.06.001
Posada-Florez, F., Z. S. Lamas, D. J. Hawthorne, Y. Chen, J. D. Evans, and E. V. Ryabov. 2021. “Pupal Cannibalism by Worker Honey Bees Contributes to the Spread of Deformed Wing Virus.” Scientific Reports 11 (1): 8989. https://doi.org/10.1038/s41598-021-88649-y
Ribière, M., B. Ball, and M. F. A. Aubert. 2008. “Natural History and Geographical Distribution of Honey Bee Viruses.” In Virology and the Honey Bee, edited by M. F. A. Aubert, B. V. Ball, I. Fries, R. F. A. Moritz, N. Milani, and I. Bernardinelli, pp. 15–84, European Commission.
Ribière, M., V. Olivier, and P. Blanchard. 2010. “Chronic Bee Paralysis: A Disease and a Virus Like No Other?” Journal of Invertebrate Pathology 103: S120–S131. https://doi.org/10.1016/j.jip.2009.06.013
Riveros, G., N. Arismendi, N. Zapata, et al. 2018. “A Scientific Note on First Detection of Kashmir Bee Virus in Apis mellifera (Hymenoptera: Apidae) in South America.” Apidologie 49:220–223. https://doi.org/10.1007/s13592-017-0545-z
Runckel, C., M. L. Flenniken, J. C. Engel, et al. 2011. “Temporal Analysis of the Honey Bee Microbiome Reveals Four Novel Viruses and Seasonal Prevalence of Known Viruses, Nosema, and Crithidia.” PLoS ONE 6 (6): e20656. https://doi.org/10.1371/journal.pone.0020656
Rueppell, O., M. K. Hayworth, and N. P. Ross. 2010. “Altruistic Self‐Removal of Health‐Compromised Honey Bee Workers from Their Hive.” Journal of Evolutionary Biology 23 (7): 1538–1546. https://doi.org/10.1111/j.1420-9101.2010.02022.x
Siede, R., and R. Büchler. 2003. “Symptomatic Black Queen Cell Virus infection of Drone Brood in Hessian Apiaries.” Berliner und Munchener tierarztliche Wochenschrift 116:130–133.
Šimenc, L., T. Knific, and I. Toplak. 2021. “The Comparison of Honeybee Viral Loads for Six Honeybee Viruses (ABPV, BQCV, CBPV, DWV, LSV3 and SBV) in Healthy and Clinically Affected Honeybees with TaqMan Quantitative Real-Time RT-PCR Assays.” Viruses 13 (7): 1340. https://doi.org/10.3390/v13071340
Stamets, P. E., N. L. Naeger, J. D. Evans, et al. 2018. “Extracts of Polypore Mushroom Mycelia Reduce Viruses in Honey Bees.” Scientific Reports 8 (1): 13936. https://doi.org/10.1038/s41598-018-32194-8
Starks, P. T., C. A. Blackie, and T. D. Seeley. 2000. “Fever in Honeybee Colonies.” Naturwissenschaften 87:229–231. https://doi.org/10.1007/s001140050709
Sumpter, D. J. T., and S. J. Martin. 2004. “The Dynamics of Virus Epidemics in Varroa-Infested Honey Bee Colonies.” Journal of Animal Ecology 73 (1): 51–63. https://doi.org/10.1111/j.1365-2656.2004.00776.x
Tentcheva, D., L. Gauthier, N. Zappulla, et al. 2004. “Prevalence and Seasonal Variations of Six Bee Viruses in Apis mellifera L. and Varroa destructor Mite Populations in France." Applied and Environmental Microbiology 70 (12): 7185–7191. https://doi.org/10.1128/AEM.70.12.7185-7191.2004
Toplak, I., U. J. Ciglenečki, K. Aronstein, and A. Gregorc. 2013. “Chronic Bee Paralysis Virus and Nosema ceranae Experimental Co-Infection of Winter Honey Bee Workers (Apis mellifera L.).” Viruses 5 (9): 2282–2297. https://doi.org/10.3390/v5092282
Traynor, K. S., F. Mondet, J. R. de Miranda, et al. 2020. “Varroa destructor: A Complex Parasite, Crippling Honey Bees Worldwide.” Trends in Parasitology 36 (7): 592–606. https://doi.org/10.1016/j.pt.2020.04.004
Wagoner, K., M. Spivak, A. Hefetz, T. Reams, and O. Rueppell. 2019. “Stock-Specific Chemical Brood Signals Are Induced by Varroa and Deformed Wing Virus, and Elicit Hygienic Response in the Honey Bee.” Scientific Reports 9 (1): 8753. https://doi.org/10.1038/s41598-019-45008-2
Wei, R., L. Cao, Y. Feng, Y. Chen, G. Chen, and H. Zheng. 2022. “Sacbrood Virus: A Growing Threat to Honeybees and Wild Pollinators.” Viruses 14 (9): 1871. https://doi.org/10.3390/v14091871
Yañez, O., N. Piot, A. Dalmon, et al. 2020. “Bee Viruses: Routes of Infection in Hymenoptera.” Frontiers in Microbiology 11:943. https://doi.org/10.3389/fmicb.2020.00943
Table 1. Common Florida honey bee viruses.