The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The family Tabanidae, commonly known as horse flies and deer flies, contains pests of cattle, horses, and humans. In Florida there are 35 species of Tabanidae that are considered economically important. Horse flies are in the genus Tabanus and deer flies are in the genus Chrysops. The yellow fly, Diachlorus ferrugatus (Fabricius), is known in Florida as a fierce biter. Like mosquitoes, it is the female fly that is responsible for inflicting a bite. The males are mainly pollen and nectar feeders. Tabanids are most likely encountered in hot summer and early fall weather. They are active during daylight hours.

Credit: Jerry Butler, University of Florida
Distribution
Horse flies and deer flies are world wide in distribution. They are, however, unreported in Hawaii, Greenland, and Iceland. In the United States, Florida produces a large population of tabanids because of the availability of suitable habitat. Florida's mild climate and large, permanently wet and undeveloped areas provide good breeding areas.
Description
Eggs
Eggs are laid in masses ranging from 100 to 1000 eggs. Eggs are laid in layers on a vertical surface, such as overhanging foliage, projecting rocks, sticks, and aquatic vegetation. Aquatic vegetation is preferred. A shiny or chalky secretion, which aids in water protection, often covers eggs. The vertical surfaces on which the eggs are deposited are always directly over water and wet ground favorable to the development of larvae. The female will not deposit egg masses on vegetation that is too dense. Eggs are initially a creamy white color but soon darken to gray and black. Eggs are cylindrical in shape and measure from 1 to 2.5 mm (~1/32 to 1/8 in) in length. Eggs hatch in five to seven days, depending upon ambient weather conditions, and the larvae fall to the moist soil and water below.

Credit: Jerry Butler, University of Florida

Credit: John Capinera, University of Florida
Larvae
Larvae use a hatching spine to break out of the egg case. The larvae are aquatic, semi-aquatic or terrestrial. Chrysops spp. are termed "hydrobionts" and are found in areas with high water content. Tabanus spp. prefer drier substrates and are "hemi-hydrobionts." The larvae taper at each end and are usually whitish in color, but also can be brownish or green depending on the species. Black bands are found around each segment of the body in many species. The larva breathes through a tracheal siphon located at their posterior end. The larva has a small head and 11 to 12 additional segments. Larvae pass through six to nine stadia. The time spent in the larval stage can last from a few months to a year. The larvae of Chrysops feed upon organic matter in the soil. Tabanus spp. feed upon insect larvae, crustaceans, and earthworms. Even though the Tabanus spp. are considered to be carnivorous and cannibalistic, reports of as many as 120 larvae per square yard have been found. The larva moves into the upper 2.5 to 5.0 cm (~1 to 2 in) of the soil, where it is drier, when it is ready to pupate. Within two days after moving to the surface the pupal stage is reached.

Credit: Jason M. Squitier, University of Florida
Pupae
The pupae are brown colored, rounded anteriorly, tapered posteriorly, and have leg and wing cases attached to the body. There is a row of spines encircling each abdominal segment. A pupal "aster" consisting of six pointed projections is located at the apex of the abdomen. The pupal stage generally lasts from two to three weeks.

Credit: Jason M. Squitier, University of Florida
Adult
The adult fly emerges from the pupal case via a slit located along the thorax of the case. In most species the males emerge before the females. After emergence of both sexes, the flies mate. Mating starts with the male pursuing the female. Mating is initiated in the air and completed on the ground. The female then deposits an egg mass and is ready to seek a host. Adult Tabanidae are large flies with broad bodies and bulging eyes. The males are easily differentiated from female flies, because eyes are contiguous in the males and widely separated in the females. The antennae are three segmented. The thorax and abdomen are covered with fine hairs. Deer flies range in length from 7 to 10 mm while horse flies are from 10 to 25 mm (~⅜ to 1 in). The deer flies are yellow to black, have stripes on the abdomen, and possess mottled wings with dark patches. Yellow flies are yellowish with the same body shape of deer flies but have dark purple to black eyes marked with fluorescent green lines. Horse flies are black to dark brown with green or black eyes. Adult deer flies have apical spurs on the hind tibiae that are not present in horse flies.

Credit: Sturgis McKeever, Georgia Southern University; https://www.insectimages.org/
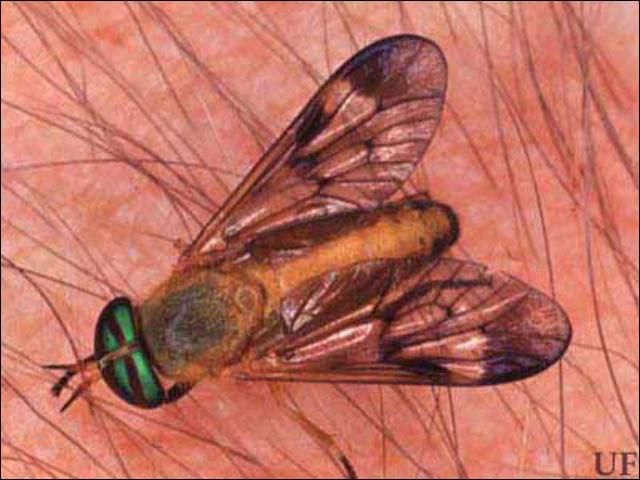
Credit: Jerry Butler, University of Florida

Credit: James Castner, University of Florida
Life Cycle
Adult tabanids are encountered in Florida between the months of May and September. Most species overwinter in the larval stage and pupate during the spring and early summer. An egg mass has been found as early as May 5 and as late October 13. Most have a yearlong life cycle, but some larger species may take two or three years. Adult life span is 30 to 60 days.
Damage
Tabanids lie in wait in shady areas under bushes and trees for a host to happen by. Sight is the main host-finding mechanism, but carbon dioxide and odor also play a role. Moving objects, especially if dark colored, are most prone to attack. Attacks occur during daylight hours with a peak beginning at sunrise and lasting three hours. A second peak is two hours before sunset and commences shortly after. Attack frequency is low on overcast days or at temperatures below 22ºC (71.6°F) and above 32ºC (89.6°F). On livestock, biting occurs on the abdomen, legs, and neck. Tabanids inflict deep wounds that cause a flow of blood. The mandibles and maxillae penetrate the skin in a scissor-like action. Anticoagulants in the saliva are pumped into the wound and the blood is ingested through the sponging labella. Pathogens may be transmitted from flies that are disturbed while feeding on one animal and begin feeding on another. It is known that deer flies can mechanically vector Tularemia and Loa loa, and horse flies transmit Anthrax. Fly attacks result in lowered gains and low milk production in livestock animals. In 1976, estimated losses in the United States were at 40 million dollars. One cattle ranch in Kentucky lost an average 100 lb. per animal due to tabanids. It is not uncommon to see as many as 100 flies feeding on an animal at one time. Twenty to thirty flies feeding for six hours are capable of taking 100 cc (3.4 fl oz)of blood.
Biological Control
There are no effective biological control programs for controlling tabanids. There are native beneficial insects that target tabanids. Eggs are parasitizied by such Hymenopteran families as Trichogrammatidae, Scelionidae, and Chalcididae. Diapriidae and Pteromalidae (Hymenoptera), and Bombyliidae and Tachinidae (Diptera) parasitize the larvae and pupa. Tabanid adults are used as provisions for nest building wasps. Cattle egrets and killdeer are also tabanid feeders.
Management
Currently there are no adequate means for managing populations. Traps are sometimes effective in small areas such as yards, camping sites, and swimming pools. Trapping of nuisance flies has reduced their numbers on the Atlantic Coast of the United States. Traps have been effective when used around cattle that are confined to manageable areas.
Some traps are black and shiny balls. The flies are attracted to these objects as the wind moves them. Malaise traps can catch large numbers of flies by simply being in their flight paths or by the use of attractants, such as CO2 and octenol. These traps are mostly useful for sampling. For personal protection, long sleeve shirts and pants in combination with a repellent containing diethyltoluamide (DEET), citronella, or geraniol are effective. For livestock, pyrethroid pour-ons function as limited repellents. Self-application methods are not effective for horse flies. Ear tags and head collars impregnated with insecticides have had success in control. For removal trapping, recent research has shown that blue cylinders (inverted cups, for example) coated with sticky material and attached to slow moving (<7 mi/hr) objects (e.g., the front of a truck or riding lawnmower) or on top of a cap worn atop a person's head are effective at reducing the abundance of these flies. See Trolling Deer Fly Trap for more information.

Credit: Jason Squitier, University of Florida

Credit: Andy Rasmussen, Florida A&M University

Credit: Jason Squitier, University of Florida

Credit: R. F. Mizell, University of Florida
Some large-scale methods such as manipulation of the habitat have been suggested. This could be done by removing unnecessary woody plants from residential areas or draining wet areas to reduce suitable breeding habitat.
The use of insecticides is generally thought of as economically unfeasible. Granular insecticides were applied to the water in the 1950s, but environmental effects were eventually considered. Spraying for the adults is also ineffective. Individual protection from adults can be obtained by using a repellent on exposed skin and clothing prior to exposure.
Selected References
Anderson JF. 1973. Biting behavior of saltmarsh deer flies (Diptera: Tabanidae). Annals of the Entomological Society of America 66: 21–23.
Borror DJ, Triplehorn CA, Johnson NF. 1992. An Introduction to the Study of Insects. Sanders College Publishing, Ft. Worth. 512 pp.
Burnet AM, Hays KL. 1974. Some influences of meteorological factors on flight activity of female horse flies (Diptera: Tabanidae). Environmental Entomology 3: 515–521.
Catts EP, Olkowski W. 1972. Biology of Tabanide (Diptera): mating and feeding behavior of Chrysops fulginosus. Environmental Entomology 1: 448–453.
Curran CH. 1934. The Families and Genera of North American Diptera. American Museum of Natural History, New York. pp. 148–149.
Essig EO. 1958. Insects and Mites of Western North America. The Macmillan Co., New York. 1050 pp.
Fairchild GB, Weems HV Jr. 1973. Doachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry Entomology Circular 139.
Fasulo TR. 2002. Bloodsucking Insects and Filth-feeding Flies. UF/IFAS. CD-ROM. SW 156.
Fasulo TR, Kern W, Koehler PG, Short DE. 2005. Pests In and Around the Home. Version 2.0. University of Florida/IFAS. CD-ROM. SW 126.
Foster CA, Renuad GD, Hays KL. 1973. Some effects of the environment on oviposition by Chrysops (Diptera: Tabanidae). Environmental Entomology 2: 1048–1050.
French FE, Hagan DL. 1995. Two-tier box trap catches Chrysops atlanticus and C. fuliginosus (Diptera: Tabanidae) near a Georgia salt marsh. Journal of Medical Entomology 32: 197–200.
French FE, Kline DL. 1989. 1-octen-3-ol, an effective trap attractant for Tabanidae (Diptera). Journal of Medical Entomology 26: 459–461.
Hansens EJ, Robinson JW. 1973. Emergence and movement of the saltmarsh deer flies Chrysops fluginosus and Chrysops atlanticus. Annals of the Entomological Society of America 66: 1215–1218.
Harwood RF, James MT. 1979. Entomology in Human and Animal Health. Macmillian Publishing Co., New York. 548 pp.
Herms WB. 1961. Medical Entomology. The Macmillan Co., New York. 582 pp.
Jones CM, Anthony DW. 1964. The Tabanidae (Diptera) of Florida. U.S.D.A. Bulletin 1295: 1–85.
Logothetis C, Schwardt HH. 1948. Biological studies on the horse flies of New York. Journal of Economic Entomology 41: 335–336.
McKeever S, French FE. 1997. Fascinating, beautiful, blood feeders. American Entomologist 43: 217–225.
Mizell RF. 1998. The trolling deer fly trap. UF/IFAS Pest Alert. No longer available online.
Pechuman LL. 1973. Horse flies and deer flies of Virginia (Diptera: Tabanidae). Virginia Research Division Bulletin 81: 1–9.
Riley WA, Johannsen OA. 1938. Medical Entomology. McGraw-Hill Book Co, Inc., New York. 483 pp.
Tashiro H, Schwart HH. 1949. Biology of the major species of horse flies of central New York. Journal of Economic Entomology 42: 269–272.
Wall R, Shearer D. 1997. Veterinary Entomology. Chapman & Hall, New York. 439 pp.
Wilson BH. 1968. Reduction of Tabanid popoulations on cattle with sticky traps baited with dry ice. Journal of Economic Entomology 61: 827–829.