Introduction
Florida's tropical environment is ideal for the establishment of numerous exotic organisms from other areas of the world. Many of the most serious insect pests and weeds in Florida are exotic and were either accidentally or intentionally introduced into the state. In the case of insects, the introductions were predominantly accidental. A few of the many examples include Formosan subterranean termite, Asian citrus psyllid, lobate lac scale, pink hibiscus mealybug, citrus leafminer, Mexican bromeliad weevil and cycad aulacaspis scale.
In contrast to exotic insects, most exotic plants were intentionally introduced into Florida, and the vast majority of these cause no significant economic or environmental harm. In fact, many of the important food and industrial crops grown in Florida are exotic, including citrus, sugarcane, corn, tomatoes and cotton. A large number of popular ornamental plants also are from other countries. Unfortunately, a small number of these introduced plants multiply, spread into natural areas and change the local ecology. These plants are called invasive, and well-known examples include Brazilian peppertree, melaleuca, Australian pine, air potato, tropical soda apple, hydrilla and water hyacinth.
One of the reasons that some exotic insects and plants become pests in their new range is a lack of natural enemies. Natural enemies include the insects, pathogens and other organisms that attack and feed on the pest in its native home. In most cases, the exotic insect or plant is not a serious pest in its native home, because its density is effectively suppressed by a complex group of natural enemies. One method that has been successfully used to control exotic insects and plants is called classical biological control. Classical biological control attempts to reunite exotic pests with their natural enemies by introducing natural enemies into the area of invasion. Examples of successful biological control in Florida include the establishment of a flea beetle and moth for control of alligatorweed (https://edis.ifas.ufl.edu/in779), an Asian parasitic wasp against citrus blackfly (https://edis.ifas.ufl.edu/in511), and two parasitic wasps from India for control of pink hibiscus mealybug (https://edis.ifas.ufl.edu/in156). Recent work on biological control of Melaleuca using insects from Australia has been highly successful (https://edis.ifas.ufl.edu/in495, https://edis.ifas.ufl.edu/in368, https://edis.ifas.ufl.edu/in1140).
Steps Involved in Classical Biological Control
Classical biological control follows the same process, regardless of whether the targeted pest is an insect or a plant. The first step is to correctly identify the exotic pest and determine its native range. Next, scientists go to the pest's native range and find organisms that attack it there. Candidate species are selected based on biological observations and brought to a containment facility for additional study. Once scientists and state and federal regulatory agencies (Florida Department of Agriculture and Consumer Services and United States Department of Agriculture, Animal Plant Health Inspection Service) gather the necessary data that clearly demonstrates that a natural enemy will cause no economic or environmental harm (https://edis.ifas.ufl.edu/in607), it is released into the environment. After release, scientists monitor the natural enemy to determine whether it has established (resulted in a self-sustaining population), and if so, measure its impact on the pest population.
The Need for Containment Facilities
After scientists release an exotic natural enemy into the environment and it becomes established, they cannot recall it. Containment is required so that scientists can thoroughly study natural enemies in a secure environment, before deciding whether they are safe to release. There are two major tasks to accomplish in containment. First, scientists must examine the natural enemies to make sure that they harbor no unwanted organisms, such as parasites or pathogens. Second, they must determine the host range of the natural enemies to make sure that they only attack the targeted pest. As society's attitudes towards environmental conservation have changed over the years, the attention given to host specificity of biological control agents has greatly increased. Today, testing of candidate biological control agents of invasive plants involves feeding studies on related native species, economically important species, and endangered/threatened species. Typically, three types of tests are conducted. First, the insects are placed on a test plant in a no-choice situation, where the insect either feeds on the plant or starves. If some feeding is observed, then multiple choice tests are performed to determine whether the insect would feed on the non-target plant if it were given a choice between the target weed and one or more non-target plants. Finally, with many insects the choice of feeding site is made by the adult female when she lays her eggs. Oviposition studies may be conducted to determine whether the insect would lay eggs on non-target plants.
Non-target testing of the natural enemies of pest insects includes studying the capability of candidate biological control agents to attack closely-related species, beneficial insects (such as honeybees and biological control agents of weeds and other pest insects) and rare and endangered fauna. Target pest insects are exposed to the natural enemies in no-choice tests and in choice tests with non-target insects. Data are then collected on the frequency of parasitization or predation on the non-intended target organisms. When all these tests are completed, which takes several years, a determination is made by the scientists about whether to go forward and request permission to release the insect. All the data are compiled and submitted to federal and state authorities for review, and they make the final decision about release.
Special Construction Requirements and Operational Procedures
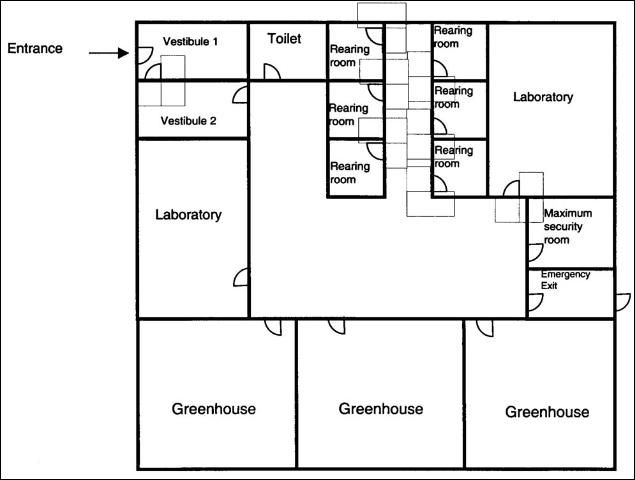
Containment facilities are carefully constructed to minimize the possibility of escape of natural enemies while they are being studied. Modern containment facilities include several features listed below to minimize the possibility of escapes. An example of a containment floor plan is found in Figure 1.
- Entrance to a containment facility is through a series of vestibules with well-sealed interlocking doors such that no two doors can be open at the same time.
- Containment areas are constantly under negative air pressure. This means that when any doors are opened, the flow of air will be into the containment area rather than towards the outside.
- All wastewater leaving the facility is sterilized with heat to kill any organisms which may enter a drain.
- All solid waste from the facility is sterilized in an autoclave at high pressure and temperature before removal.
- Either there are no windows, or if windows do exist, they are permanently sealed and are high strength (double-paned or Lexan).
- Air leaving the facility passes through very fine high efficiency particulate air (HEPA) filters.
- There are no perforations in the walls. All electrical conduits, lights, outlets and switches are mounted on the wall, rather than in the wall as is typical in normal construction. All perforations in the ceilings and floors, as well as all joints between walls, ceilings and floors, are sealed with silicone.
- The operation of each containment facility is monitored by a quarantine officer or containment director who is responsible for the physical integrity of the construction and for making certain that scientists and staff working in the facility follow operating procedures.
- Shipments received directly from overseas are opened in a maximum-security room by the quarantine officer and the scientist leading the project. Plant material, undesirable insects and packing material are immediately destroyed in an autoclave. Import permits are checked and a record of what and how many natural enemies were received is filed.
- Access to containment areas is limited to only those scientists and technicians who work in the facility and have been trained on containment operating procedures.
Credit: UF/IFAS
The two facilities in Fort Pierce and Fort Lauderdale (described below) are both constructed to withstand category III hurricanes. If a category III hurricane is predicted, all quarantined organisms will be moved from greenhouses to the central sections of the buildings. A stronger hurricane would require that all quarantined organisms are destroyed or moved to another approved facility (time permitting).
Biological Control Containment Facilities in Florida
Until very recently, there were only two permitted arthropod containment facilities in Florida, both located in Gainesville. The oldest facility, which opened in 1973 and was one of the first facilities in the United States, is operated by the Division of Plant Industry (DPI) of the Florida Department of Agriculture and Consumer Services. It provides services for the University of Florida, the Division of Plant Industry and the United State Department of Agriculture, Agricultural Research Service (USDA/ARS). A second smaller facility is operated by the University of Florida's Department of Entomology and Nematology. Due to size limitations of the two facilities in Gainesville, the demand for containment space in Florida was not met. Fortunately, two additional state-of-the-art containment facilities were opened in 2004. The USDA/ARS Invasive Plant Research Laboratory in Fort Lauderdale constructed a 21,000 sq. ft. facility (Figure 2), and the University of Florida and DPI opened a similar 17,000 sq. ft. facility in Fort Pierce (Figure 3). Currently, scientists at the Fort Lauderdale facility are working on biological control of melaleuca, old-world climbing fern, Brazilian peppertree, Chinese tallow, , water hyacinth, and other exotic pests. The Fort Pierce faculty is addressing invasive weeds (Brazilian peppertree, air potato, water hyacinth, earleaf acacia) and insects/arthopods (Asian citrus psyllid, spherical mealybug, ghost bulimulus). These containment laboratories have greatly expanded opportunities for conducting classical biological control research and implementation in Florida and work together to provide sustainable control for some of Florida’s worst invasive species.

Credit: UF/IFAS

Credit: UF/IFAS