The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids, and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The predatory gall midge, Feltiella acarisuga (Vallot), is one of the most effective and widespread natural enemies of spider mites (Tetranychidae) (Gagné 1995). Because of their flying and prey-detecting capabilities, and high feeding potential, it is considered an important natural enemy of the twospotted spider mite, Tetranychus urticae Koch, in a number of cropping systems (Opit et al. 1997; Refaei and Mohamed 2013). It is also known to feed on other pest mites, including brown almond mite, Bryobia rubrioculus Scheuten; carmine spider mite, Tetranychus cinnabarinus Boisduval; and European red mite, Panonychus ulmi Koch. Feltiella acarisuga could be particularly useful for integrated pest management of spider mites that attack greenhouse crops (Gillespie et al. 1998).
Synonymy
Cecidomyia acarisuga
Mycodiplosis minuta
Therodiplosis persicae
Therodiplosis beglarovi
Arthrocnodax rutherfordi
Feltiella tetranychi
Feltiella davisi
Feltiella americana
Feltiella ithacae
Feltiella quadrata (Gagné 1995)
Distribution
The genus Feltiella is virtually cosmopolitan and contains 10 species: Feltiella acarisuga (worldwide, except for the Neotropical Region), Feltiella pini (Felt) (North and Central America, West Indies, Australia), Feltiella curtistylus Gagné (Brazil), Feltiella occidentalis (Felt) (US—California; Japan—Honshu), Feltiella acarivora (Zehnter) (Indonesia- Java; Australia), Feltiella insularis (Felt) (US—Illinois, New Jersey, Florida; West Indies, Colombia, Argentina), Feltiella reducta Felt (US—New York), Feltiella ligulata Gagné (Cape Verde Island), Feltiella kanchanjungaensis (India—West Bengal) and Feltiella tetranychi (Germany) (Gagné 1995, 2010). Feltiella acarisuga is the most widely distributed species in the genus and is listed from the US, Canada, Finland, Germany, United Kingdom, Switzerland, Italy, Morocco, Greece, Israel, India, Sri Lanka, Taiwan, Japan, and New Zealand. It is the only species of Feltiella found throughout most of Europe and Asia.
Description
Egg
The glossy, translucent, oblong eggs are deposited individually near prey mites on leaves. They are 0.1 x 0.25 mm in size (Koppert 1997). The eggs hatch ~ 2.5 days after oviposition, and the larvae immediately begin to feed (Mo and Liu 2007).
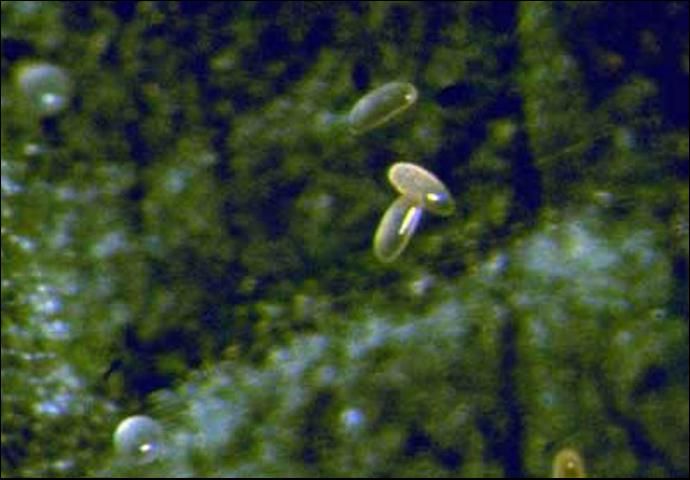
Credit: David R. Gillespie, Agassiz
Larva
The orange-brown larvae vary in length from 0.2 to 2 mm during their four developmental instars (Koppert 1997). They forage for mites on leaves and feed for four to six days, depending on temperature, relative humidity and the abundance of prey mites (Gillespie and Raworth 1999). They feed exclusively on all developmental stages of several species of spider mites. Feltiella acarisuga larvae occur in populations as large as 160 per cm2 of eggplant leaf.

Credit: Lance S. Osborne, UF/IFAS
Pupa
The fluffy white, 1 to 1.5 mm long pupa requires four to six days to complete development and produce an adult (Koppert 1997). Pupation occurs mainly on the underside of a leaf next to a vein.

Credit: Lance S. Osborne, UF/IFAS
Adult
The adult Feltiella acarisuga is a delicate, pink-brown fly about 2 mm in length with long legs (Koppert 1997). Adults live for about 12–14 days, and on average, a single female lays about 33 eggs in its life span at the rate of 3.2 eggs/oviposition day. Males have a shorter life span than females (Mo and Liu 2007). The sex ratio is about 1:1. Adult Feltiella acarisuga are not predaceous but drink water and nectar.

Credit: David R. Gillespie, Agassiz
Life Cycle
In climates without extremely dry or cold seasons, every stage of Feltiella acarisuga is present year-round. Feltiella spp. apparently develop from egg to egg in 26 to 33 days, averaging around 29 days (Sharaf 1984). Feltiella acarisuga developmental time may range between two to four weeks (Gillespie et al. 1998; Mo and Liu 2007; Refaei and Mohamed 2013) depending upon the temperature and/or relative humidity. Reproduction and development occur at 15°C–25°C. Eggs and larvae do not survive above 30°C or below 30% relative humidity. At least 50% relative humidity is required for a normal rate of development. The optimum temperature and relative humidity combination is about 20°C and 90% relative humidity. However, with an abundance of prey, the level of predation remains constant over the developmental range of temperature and relative humidity (Gillespie et al. 1998). If prey populations are sub-optimal, larvae can survive by pupating at a smaller size. Larvae also can survive for several days without prey.
Effectiveness
Feltiella acarisuga can be used to manage spider mite populations in a variety of greenhouse and field crops, especially when incorporated into a bio-intensive IPM program. In eggplant, for example, Feltiella acarisuga has appeared naturally and reduced spider mite numbers by more than 40% (Sharaf 1984). Each midge larva can consume an average of least 15 adult mites, 30 mixed developmental stages, or 80 eggs per day. In a recent laboratory study, Xiao et al. (2012) reported Feltiella acarisuga had higher predation capacity than two phytoseiid predators Neoseiulus californicus (McGregor) and Amblyseius swirskii (Athias-Henriot) on Tetranychus urticae eggs. The maximum daily predation observed by a second instar larval Feltiella acarisuga was 50 eggs/day, compared to 25.6 eggs/day and 15.1 eggs/day consumed by a female Neoseiulus californicus and a female Amblyseius swirskii, respectively. In a related study, modified corn banker plant system using Oligonychus pratensis (Banks) as a surrogate host for dispersal and predation potential of Feltiella acarisuga against Tetranychus urticae on beans was evaluated (Xiao et al. 2011). The system showed promising results, where within two weeks period, Feltiella acarisuga was found to fly at least 7.0 m far from the corn-banker plants in search of new prey and successfully established on the bean plants infested with Tetranychus urticae. More than 176 Feltiella acarisuga larvae per bean leaf were recorded after dispersal from the corn plant and were effective in suppressing Tetranychus urticae populations.
Weekly releases of 1000 individuals per ha have been extremely effective for controlling spider mites on tomato, pepper, and cucumber (Gillespie et al. 1998). In addition, Feltiella acarisuga (sold as Therodiplosis persicae) is being used to manage spider mites on strawberries and various ornamental crops. It is recommended that 200 to 1000 individuals per ha be released weekly as a trial rate for growers. The weekly release rate is approximately doubled for heavy infestations, 2,500 adults per ha for six successive weeks (Biobest 1999). It is highly advised that Feltiella acarisuga be released in combination with the predaceous mite, Phytoseulus persimilis, a well-established natural enemy used to control spider mites. Feltiella acarisuga is more mobile as an adult than is the predatory mite and, once established, eats at least five times as many spider mites (Biobest 1999). However, Phytoseulus persimilis should not be released where Feltiella acarisuga is becoming established because they are known to eat midge eggs if prey is limited (Gillespie 1998). Feltiella acarisuga has also been found to be active in cool and dark weather during spring and autumn seasons. Thus, it can be used for spider mite control in strawberry and other crops that requires low temperature for production (Biobest 1999).
Commercial Availability and Use
Feltiella acarisuga pupae are commercially available from several producers and suppliers of natural enemies (http://www.anbp.org/). Pupae are shipped on leaves or an inert substance in various containers, such as 1-liter pots. Pots are placed in the crop on the ground at the beginning of rows and their lids are pierced to release the adult midges. It is best if the midges are released near concentrations of spider mites. To establish, Feltiella acarisuga requires fairly large prey populations (Gillespie and Raworth 1999). The pots should be stored in the dark for no more than two days at 10 to 15°C (Koppert 1997). Adults should be released from containers every 24 hours, late at night or early in the morning because of the cooler and more humid conditions. The RH should be kept above 80%, if possible (Gillespie and Raworth 1999).
It is essential to avoid non-target side effects of chemical pesticides, such as Thiodan, Diazinon, and Kelthane; however, most fungicides are safe to use with Feltiella acarisuga (Gillespie and Raworth 1999). Sulfur products used as dusts or sprays do not cause mortality in larvae but females avoid laying eggs on treated plants. Among commonly used insecticides in horticultural and field production, foliar application of azadiractin, flonicamid, pyriproxifen and spinosad, and drench applications of imidacloprid are reported to have no toxic effect on Feltiella acarisuga population (mortality <25%), whereas pymetrozine and buprofezin had no to moderate impact (mortality <50%) (Biobest 2012). Abamectin, bifenazate, bifenthrin, cyfluthrin, chlorfenapyr, esfenvalarate, permethrin and spiromesifen were found to be toxic (mortality >75%) against this predator. Another concern is parasitization of Feltiella acarisuga larvae by Aphanogmus floridanus, potentially a very abundant parasitoid during warmer months. However, if necessary, releases can be timed to avoid the parasitoid because unlike the parasitoid Feltiella acarisuga does not diapause during the cooler months. Feltiella acarisuga parasitized by Aphanogmus floridanus have pupal cases with characteristic round emergence holes.
Selected References
Biobest NV. (1999). Therodiplosis persicae. Belgium. https://www.biobestgroup.com/ (28 January 2019)
Biobest NV. (2012). Side effect manual. https://www.biobestgroup.com/en/side-effect-manual (28 January 2019)
Gagné RJ. 1995. "Revision of tetranychid (Acarina) mite predators of the genus Feltiella (Diptera: Cecidomyiidae)." Annals of the Entomological Society of America 88: 16–30.
Gagné RJ. (2010) Update for a catalog of the Cecidomyiidae (Diptera) of the world. Digital version 1. http://www.ars.usda.gov/SP2UserFiles/Place/12754100/Gagne_2010_World_Catalog_Cecidomyiidae.pdf (July 5 2016).
Gillespie DR, Raworth DA. 1999. Biological Control of Twospotted Spider Mites on Greenhouse Vegetable Crops. Agriculture and Agri-Food Canada. P. 30–32.
Gillespie DR, Roitberg B, Basalyga E, Johnstone M, Opit G, Rodgers J, Sawyer N. 1998. Biology and application of Feltiella acarisuga (Vallot) (Diptera: Cecidomyiidae) for biological control of twospotted spider mites on greenhouse vegetable crops. Pacific Agri-Food Research Centre (Agassiz)Technical Report, No. 145. Agriculture and Agri-Food Canada.
Koppert BV. 1997. SPIDEND. Feltiella acarisuga. Netherlands. http://www.koppert.nl/ (10 August 2012).
Mo TL, Liu TX. 2007. "Predation and life table of (Diptera: Cecidomyiidae) preying on eggs of (Acari: Tetranychidae)." Environmental Entomologist 36: 369–375.
Opit GP, Roitberg B, Gillespie DR. 1997. "The functional response and prey preference of Feltiella acarisuga(Vallot) (Diptera: Cecidomyiidae) for two of its prey: male and female twospotted spider mites, Tetranychus urticae Koch (Acari: Tetranychidae)." Canadian Entomologist 129: 221–227.
Osborne LS, Ehler LE, Nechols JR. (July 1999). Biological control of the twospotted spider mite in greenhouses.(10 August 2012).
Refaei GS, Mohamed AA. 2013. Biological characters of Feltiella acarisuga (vallot) (Diptera: Cecidomyiidae) when fed on eggs of Tetranychus urticae koch [Acari: Tetranychidae]
Sharaf NS. 1984. "Studies on natural enemies of tetranychid mites infesting eggplant in the Jordan Valley." Zeitschrift für Angewandte Entomologie 98:527–533.
Xiao YF, Osborne L, Chen JJ, McKenzie C, Houben K, Irizarry F. 2011. "Evaluation of corn plant as potential banker plants for predatory gall midge, Feltiella acarisuga (Diptera: Cecidomyiidae) against Tetranychus urticae (Acari: Tetranychidae) in greenhouse vegetable crops." Crop Protection 36:1635–1642.
Xiao YF, Osborne L, Chen JJ, McKenzie C. 2012. "Functional responses and prey-stage preferences of a predatory gall midge and two predacious mites with twospotted spider mites, Tetranychus urticae, as host." Journal of Insect Science 13:8. Available online: http://www.insectscience.org/13.8 (05 July 2016).